1109
Diffusion-Tensor-Imaging MR-Neurography for the detection of polyneuropathy in Type 1 diabetes1Dept. of Neurology, Aarhus University Hospital, Aarhus C, Denmark, 2Dept. of Neuroradiology, Würzburg University Hospital, Würzburg, Germany, 3MR Research Centre, Aarhus University Hospital, Aarhus N, Denmark, 4Dept. of Neurophysiology, Aarhus University Hospital, Aarhus C, Denmark, 5Heidelberg University Hospital, Heidelberg, Germany, 6Dept. of Endocrinology, Aarhus University Hospital, Aarhus C, Denmark
Synopsis
Diabetic peripheral neuropathy (DPN) is an irreversible complication that often remains undiagnosed until advanced stages. Thus, to prevent progression of DPN, early diagnosis is important emphasizing the need for more sensitive diagnostic techniques. Diagnosis is based on a neurological examination, nerve conduction studies, and quantitative sensory testing. Magnetic-resonance-neurography (MRN) enables microstructural nerve fascicle imaging of peripheral neuropathies (1–4). In a previous study on patients with severe DPN we found that diffusion-tensor-imaging (DTI) is a reproducible method in detection of DPN (5). Furthermore, DTI-MRN provided a more accurate group separation than by changes based on T2 or proton-density contrast.
Synopsis
Diabetic peripheral neuropathy (DPN) is an irreversible complication that often remains undiagnosed until advanced stages. Thus, to prevent progression of DPN, early diagnosis is important emphasizing the need for more sensitive diagnostic techniques. Diagnosis is based on a neurological examination, nerve conduction studies, and quantitative sensory testing.
Magnetic-resonance-neurography (MRN) enables microstructural nerve fascicle imaging of peripheral neuropathies (1–4). In a previous study on patients with severe DPN we found that diffusion-tensor-imaging (DTI) is a reproducible method in detection of DPN (5). Furthermore, DTI-MRN provided a more accurate group separation than by changes based on T2 or proton-density contrast.
Purpose
To evaluate if diffusion-tensor-imaging MR-Neurography (DTI-MRN) of the sciatic and tibial nerves can be used for detection and staging of diabetic peripheral neuropathy in patients with type 1 diabetes. Furthermore, applying receiver operating characteristic (ROC) analyses we aimed to determine the sensitivity and specificity of the MR methodology.Methods
Forty-nine type 1 diabetic patients (11 with severe polyneuropathy (sDPN), 13 with mild/moderate polyneuropathy (mDPN) and 25 without polyneuropathy (nDPN)) and 30 healthy controls (HC) were included. Clinical examinations, nerve conduction studies and vibratory-perception-thresholds, determined the presence and severity of DPN.
MR examinations were performed on a 3T MR-scanner (Skyra, Siemens AG, Erlangen, Germany) using a 15-channel send/receive Knee Coil (Siemens AG, Erlangen, Germany). MR images were acquired at the left leg at predetermined locations including proximal (sciatic nerve) and distal regions of the lower extremity (tibial nerve). The MR protocol consisted of spin-echo (SE) images with 10 different echo times (TR = 3280 ms, TE1-10 = [13, 25, 38, 51, 63, 76, 89, 101, 114, 127] ms, FOV 160x160 mm2, matrix size 512x512, voxel size 0.3x0.3x3 mm3, 2 averages) to calculate T2 relaxometry and proton-spin-density and diffusion-weighted-images (TR/TE 4200/112 ms, b = [0, 800] s/mm2, directions = 12, 4 averages, FOV 175x175 mm2, matrix size 128x128, voxel size 1.36x1.36x3 mm3) for calculation of diffusion parameters (apparent diffusion coefficient (ADC), fractional anisotropy (FA) and trace). Analyses of ADC and FA images were performed with nerve segmentation based on the trace images. The net imaging time was 25 min 40 sec at each location with coil reposition requiring additionally 5 min. The MR sequences consisted of 16 axial slices and with a strong fat suppression pulse to reduce inter-fascicular fat.
Results
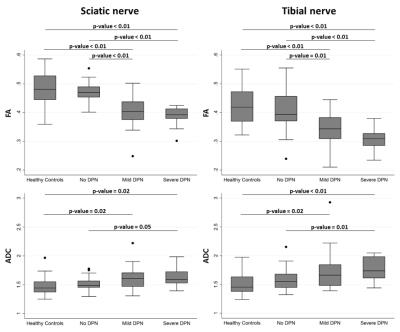
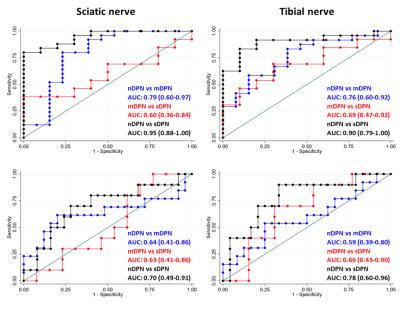
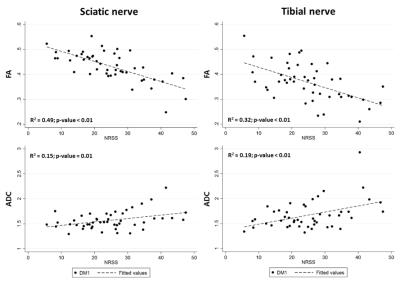
DTI-MRN could accurately discriminate between DPN, nDPN and HC. The proximal FA was lowest in sDPN (sDPN 0.38±0.04; mDPN 0.41±0.07; nDPN 0.47±0.02; HC 0.48±0.06; p<0.01). In addition, distal FA was lowest in sDPN (sDPN 0.31±0.08; mDPN 0.34±0.06; nDPN 0.41±0.07; HC 0.42±0.07; p<0.01). Likewise, proximal ADC was highest in sDPN with lower values in patients with mPDN and nDPN as well as in HC (Figure 1). Receiver operating characteristic (ROC) curves showed high diagnostic accuracy in for group separation indicated by area under the curve values of the sciatic nerve (FA 0.60-0.95 ; ADC 0.63-0.70) and tibial nerve (FA: 0.69-0.90 ; ADC: 0.59-0.78) (Figure 2). The severity of neuropathy was correlated to DTI-MRN demonstrating a strong association of proximal (FA: R2 = 0.49, p<0.01; ADC: R2 = 0.15, p = 0.01) and distal nerve lesions (FA: R2 = 0.32, p< 0.01; ADC: R2 = 0.19; p-value < 0.01) (Figure 3). T2 relaxometry and proton-spin-density did not enable detection of nerve lesions.Discussion
MRN is able to detect nerve abnormalities in patients with type 1 diabetes closely related to the severity of neuropathy suggesting that MRN can be used to detect structural signs of neuropathy. We extended previous studies investigating MRN in DPN (2, 4) by exclusively investigating DPN of type 1 diabetes patients in this study, and by incorporating DTI, for the first time. Our main finding is the superior diagnostic accuracy of quantitative DTI over proton-spin-density or T2 contrast. In line with previous studies, we found that the structural nerve alterations as visualized by imaging appear most marked at the proximal rather than the distal level (2, 4).Conclusions
In conclusion, we found close associations between findings of DTI-MRN and the presence and severity of neuropathy by decreasing nerve FA and increasing ADC in proximal and distal nerve segments of patients with type 1 diabetes. These alterations are likely to reflect proximal and distal nerve fiber pathology.Acknowledgements
The authors acknowledge Søren Gregersen (Department of Endocrinology and Internal Medicine, Aarhus University Hospital) for contributing to patient recruitment.
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is funded by the UNIK partnership foundation, Siemens A/G Copenhagen, the Danish Diabetes Academy supported by the Novo Nordisk Foundation, Aarhus University and the BEVICA Foundation. MP was supported by the Deutsche Forschungsgemeinschaft (SFB 1158, Project A3). SH was supported by the Deutsche Forschungsgemeinschaft (SFB 1118 Project, B05).
References
1. Chhabra A, Andreisek G, Soldatos T, et al.: MR neurography: past, present, and future. AJR Am J Roentgenol 2011; 197:583–91.
2. Pham M, Oikonomou D, Bäumer P, et al.: Proximal neuropathic lesions in distal symmetric diabetic polyneuropathy: findings of high-resolution magnetic resonance neurography. Diabetes Care 2011; 34:721–3.
3. Kollmer J, Hund E, Hornung B, et al.: In vivo detection of nerve injury in familial amyloid polyneuropathy by magnetic resonance neurography. Brain 2015; 138(Pt 3):549–62.
4. Pham M, Oikonomou D, Hornung B, et al.: Magnetic resonance neurography detects diabetic neuropathy early and with proximal predominance. Ann Neurol 2015; 78:939–948.
5. Vaeggemose M, Pham M, Ringgaard S, et al.: Diffusion Tensor Imaging MR Neurography for the Detection of Polyneuropathy in Type 1 Diabetes. J Magn Reson Imaging 2016:1–10.
Figures