1108
Imaging of the Brachial Plexus using a 3D Dixon-TSE Pulse Sequence with Blood Vessel and CSF Signal Suppression: Preliminary Experience in Children1Department of Diagnostic and Interventional Radiology, Klinikum rechts der Isar, Technische Universitat Munchen, Munich, Germany, 2Department of Radiology, Phoenix Children's Hospital, Phoenix, AZ, United States, 3Department of Neuroradiology, Klinikum rechts der Isar, Technische Universitat Munchen, Munich, Germany
Synopsis
We demonstrate the clinical feasibility and utility of a 3D Dixon TSE sequence in imaging the brachial plexus nerves in children. The employed high spatial resolution MR Neurography (MRN) technique utilizes a refocusing flip angle train to maximize nerve signal and to suppress cerebrospinal fluid signal. Additionally, a T2-preparation with motion-sensitizing gradients was employed to suppress flowing blood signal and Dixon-based chemical-shift water-fat imaging was used to suppress fat signal in the head, neck, and chest. We illustrate this MRN technique in delineating the brachial plexus nerves and associated pathological conditions in 25 pediatric patients (age range: 6 months-21 years).
Purpose and Introduction
There has been growing interest in high-resolution MR neurography (MRN) exams of the brachial plexus to complement the diagnosis of neurological pathologies. The brachial plexus is a complex network that comprises of the four cervical (C5-C8) and the first thoracic nerve (T1). These nerves supply afferent and efferent fibers to the chest, shoulder, and upper extremities. 3D TSE sequences combined with an improved Motion Sensitized Driven Equilibrium (iMSDE) preparation have been proposed for lumbar and brachial plexus imaging with vessel signal suppression [1-4]. Recently, Cervantes, et al., using extended phase graph analysis, optimized the 3D TSE refocusing flip angle train to improve the delineation of small lumbar plexus nerves [5] and showed that the optimized train can further reduce cerebrospinal fluid (CSF) signal when sub-millimeter resolution voxels are used [6]. These works have also shown that spectral adiabatic inversion recovery (SPAIR) was adequate in providing uniform fat suppression in lumbar plexus imaging. In this work, we modify the previous 3D TSE MRN sequence [5] for brachial plexus imaging, and showcase our experience in 25 children (age range: 6 months-21 years), some with suspected nerve pathology. Since the brachial plexus region is highly sensitive to B0 inhomogeneity, we employ a two-echo chemical-shift-encoded water-fat Dixon method to achieve uniform fat suppression [7].Materials and Methods
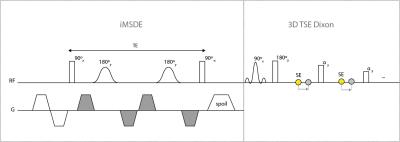
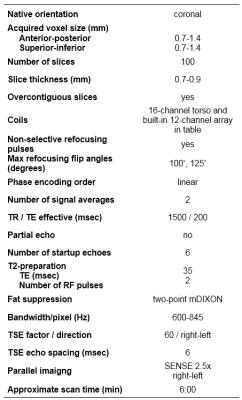
Figure 1 shows the pulse sequence diagram of the iMSDE-prepared 3D two-point Dixon TSE sequence. Figure 2 summarizes the typical imaging parameters used for brachial plexus imaging in children. The two echoes needed for Dixon water-fat separation were acquired in separate TRs. In contrast to the previously developed lumbar protocol [5, 6], the brachial plexus technique herein used a lower TSE factor and a nerve signal ratio equal to 1 (nerve signal constant for 50% of the flip angle train duration) in order to maintain adequate nerve signal, as the requisite Dixon echo sampling lengthened the echo spacing in the 3D TSE MRN sequence.
We imaged the brachial plexus of 25 pediatric patients (12 males, 13 females, 8.1±6.0 years). All patients were referred for a clinically-indicated MRI exam of the head and neck, cervical spine, or total spine. All imaging was implemented on two similar 3 Tesla platform (Philips Ingenia) using a 32-channel head coil array and a 12-channel posterior spine coil built into the MRI table. Volume-based B0 shimming of the imaging region was performed by the operator.
Two pediatric neuroradiologists in consensus assessed the conspicuity of the brachial plexus nerves in the resultant data, including the rami and the nerve roots, trunks, divisions, and cords. A three-point scale was used: “0-not visible or poorly visualized”, “1-moderately visualized”, “2-well visualized”. The reviewers also commented on the presence of artifacts and blurring, the quality of fat suppression, and the degree of blood and CSF suppression.
Results
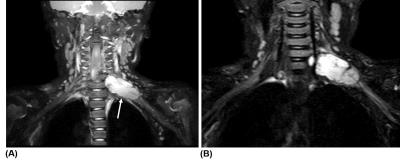
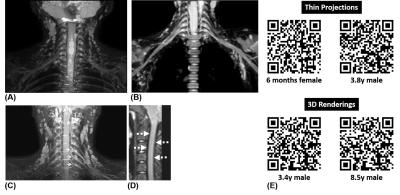
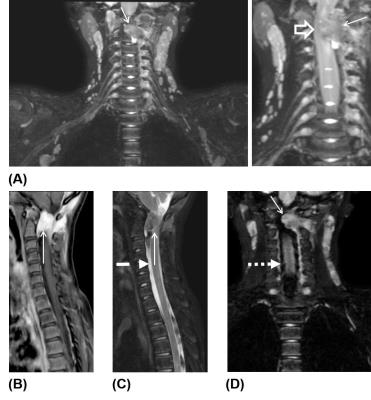
Several representative cases are shown in Figures 3-5, highlighting the diagnostic utility of the 3D TSE MRN technique. Note the consistent and uniform suppression of signals from fat, CSF, and vessels, the strong signal contrast between the brachial plexus nerves and surrounding tissue, and the clear visualization of nerve structures. Image evaluation identified one case with poor fat suppression that significantly impacted nerve visualization. The failure of fat suppression occurred along the left shoulder and arm of an 8-months-old patient. Four additional cases (age: 11 months, 3.6y, 16.5y, 21y) were rated as having poor diagnostic image quality due to improper image planning (e.g., limited FOV). These cases received a score of “0”, due primarily to their low SNR and poor nerve-background CNR. In the remaining 20 cases that were deemed diagnostically useful, 14 cases received a score of “2”, while 6 cases received a score of “1”.Discussion and Conclusion
A blood and CSF-signal suppressed 3D Dixon TSE sequence that enables clear delineation of the brachial plexus nerves has been demonstrated in children, with promising data supporting clinical adoption. The employed iMSDE preparation achieved good suppression of the large thoracic blood vessels in agreement with previous works [2, 8]. The optimized flip angle train maximized nerve signals without inducing blurring due to T2-decay apodization of signals in k-space [5], and enabled the suppression of pulsating CSF signal in all cases [6]. The two-echo Dixon approach enabled uniform fat suppression in the head-neck region, without the SNR penalty associated commonly with inversion-recovery TSE (i.e., STIR) sequences [4, 9]. In conclusion, the brachial plexus imaging technique proposed in this work can assist in the diagnostic assessment of neonates, infants, children who have birth-related injuries [10], traumatic cervical spine injuries from trauma, genetic disorders such as neurofibromatosis, and cervical spine tumors.
Acknowledgements
All authors thank Philips Healthcare for research and funding support.References
[1] Chhabra A, Thwait GK, Soldatos T, Thakkar RS, Del Grande F, Chalian M, Carrino JA. High-resolution 3T MR neurography of the brachial plexus and its branches, with emphasis on 3D imaging. Am J Neuroradiol 2013; 34:486-497.
[2] Cortes C, Ramos Y, Restrepo R, Restrepo JA, Grossman JAI, Lee E. Practical magnetic resonance imaging evaluation of peripheral nerves in children: magnetic resonance neurography. Radiol Clin N Am 2013; 51:673-688.
[3] Yoneyama M, Takahara T, Kwee TC, Nakamura M, Tabuchi T. Rapid high resolution MR neurography with a diffusion-weighted pre-pulse. Magn Reson Med Sci 2013; 12:111-119.
[4] Kasper JM, Wadhwa V, Scott KM, Rozen S, Xi Y, Chhabra A. SHINKEI--a novel 3D isotropic MR neurography technique: technical advantages over 3DIRTSE-based imaging. Eur Radiol 2015; 25:1672-1677.
[5] Cervantes B, Bauer JS, Zibold F, Kooijman H, Settles M, Haase A, Rummeny EJ, Wörtler K, Karampinos DC. Imaging of the lumbar plexus: optimized refocusing flip angle train design for 3D TSE. J Magn Reson Imaging 2016; 43:789-799.
[6] Cervantes B, Hu HH, Pokorney A, Weidlich D, Kooijman H, Rummeny E, Haase A, Kirschke JS, Karampinos DC. CSF-free imaging of the lumbar plexus using sub-millimeter resolution with 3D TSE. Proc Int Soc Magn Reson Med 2016; 24: abstract #2250.
[7] Eggers H, Brendel B, Duijndam A, Herigault G. Dual-echo Dixon iamging with flexible choice of echo times. Magn Reson Med 2011; 65:96-107.
[8] Yoneyama M, Tanji H., Yamaki T, Takahashi D, Obara M, Okuaki T, Van Cauteren M. MR NeuroAngiography: Simultaneous acquisition of brachial plexus MR neurography and subclavian MR angiography using phase-cycling. Proc Int Soc Magn Reson Med 2016; 24: abstract #368.
[9] Viallon M, Vargas MI, Jlassi H, Lövblad KO, Delavelle J. High-resolution and functional magnetic resonance imaging of the brachial plexus using an isotropic 3D T2 STIR (Short Term Inversion Recovery) SPACE sequence and diffusion tensor imaging. Eur Radiol 2008; 18:1018-1023.
[10] Smith AB, Gupta N, Strober J, Chin C. Magnetic resonance neurography in children with birth-related brachial plexus injury. Pediatr Radiol 2008; 38:159-163.
Figures