1106
Cerebral Sodium (23Na) Magnetic Resonance Imaging in Patients with Migraine1Institute of Clinical Radiology and Nuclear Medicine, University Medical Center Mannheim, University of Heidelberg, Mannheim, Germany, 2University of Bremen, MR-Imaging and Spectroscopy, Faculty 01 (Physics/Electrical Engineering), Bremen, Germany, 3Clinic for Anaesthesiology and Operative Intensive Care, University Medical Center Mannheim, University of Heidelberg, Mannheim, Germany, 4Computer Assisted Clinical Medicine, University Medical Center Mannheim, University of Heidelberg, Mannheim, Germany, 5Institute of Diagnostic and Interventional Radiology, University Hospital Cologne, Cologne, Germany
Synopsis
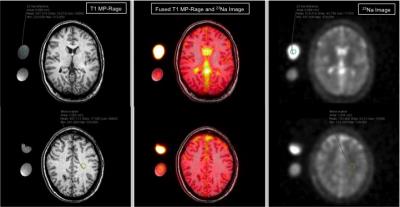
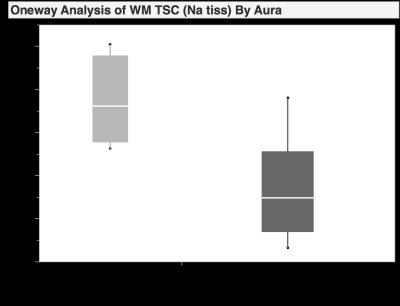
To evaluate sodium concentrations in subgroups of patients with clinically manifest migraine, 12 patients underwent a cerebral 23Na-magnetic resonance imaging examination using a dual-tuned (23Na/1H), dedicated head-coil. 23Na-sequences were reconstructed according to a T1 MP-RAGE, allowing direct cross-referencing of predetermined regions-of-interest (ROI). Significant differences in sodium concentrations could be observed for the white matter and anterior cerebrospinal fluid in patients with and without accompanying aura (p<0.05). These data suggest, that cerebral sodium concentrations may have the potential to differentiate between different subgroups of migraine.
Purpose
Migraine is one of the most common headache disorders with a prevalence of about 18% in women and 6% in men. 20% of all patients with migraine report aura-symptoms. A recent study by Harrington et. al [1] of a rat migraine model supports a role of sodium in migraine, with rising sodium levels that increase neuronal excitability. The feasibility of cerebral 23Na-MRI for in-vivo imaging has been demonstrated in several studies, including imaging in stroke, brain tumors and multiple sclerosis [2-4]. So far, no study has been published that investigated the impact of sodium concentrations in patients with migraine. Thus, the purpose of this study was the evaluation of sodium concentrations in subgroups of patients with clinically manifest migraine.Methods
In this prospective, IRB-approved study we recruited 12 otherwise healthy patients (all female; mean age 34±11 years) who had been diagnosed for migraine symptoms according the criteria of the International Headache Society (IHS). The patients filled out a questionnaire regarding onset of disease, length, intensity (scale 0 -10) and frequency of migraine attacks and accompanying aura. The patients underwent a cerebral 23Na-magnetic resonance imaging examination at 3.0T (MAGNETOM Tim Trio, Siemens Healthcare Sector, Erlangen, Germany). For each scan a non-contrast enhanced T1w MP-RAGE sequence for anatomical referencing and a 3D-density-adapted, radial gradient echo (GRE-) sequence [5] for 23Na-imaging were acquired using a dual-tuned (23Na/1H), dedicated head-coil (Rapid Biomedical GmbH, Rimpar, Germany). The following scan parameters were used: TR 120msec; TE 0.2 msec; 17000 projections; flip angle 87°; spatial resolution 3.6x3.6x3.6mm3. 23Na-sequences were reconstructed according to the MP-RAGE, allowing direct cross-referencing of regions-of-interest (ROI). Circular ROIs were placed in predetermined anatomic regions: cerebrospinal fluid (CSF), grey and white matter (GM/WM), brain stem and cerebellum. External sodium reference phantoms with 5% and 2% agar gel and sodium concentrations of 154 millimoles and 50 millimoles were used to calculate the tissue sodium concentrations (TSC). Kendall Tau and Wilcoxon rank sum test were used for statistical analysis (JMP 10.0 SAS Institute Inc., Cary, North Carolina, USA).Results
Significant differences in sodium concentration could be observed for the WM and anterior CSF in patients with and without accompanying aura (p<0.05). Moderate to good correlation was observed between time interval to last attack and anterior CSF and disease onset with TSC in GM, posterior CSF, brain stem and cerebellum (r ≥ 0.4). Furthermore, pain intensity and TSC in GM, CSF and brainstem showed a moderate correlation (r = 0.4-0.5). Overall sodium concentration of all patients (in millimoles per liter) averaged 35±4, 41±3, 81±8, 87±6 and 33±4 and 33±3 for WM, GM, anterior and posterior CSF, brainstem and cerebellum, respectively.Discussion
The diagnosis of migraine in a clinical setting is challenging, because intra-individual migraine characteristics and type of attacks vary among patients. The diagnostic criteria of the IHS and further questionnaires are helpful in most cases. Nonetheless, many migraine patients are undiagnosed and therefore undertreated. In contrast, there are patients treated incorrectly with medication against migraine who suffer from other types of headache, e.g. tension-type headache. Therefore, it would be helpful to have a diagnostic tool supporting or even diagnosing migraine and differentiating migraine from other types of headaches. Knowing the distribution of sodium concentrations in brain structures for different subgroups of migraine may have the potential of providing a more objective clinical evaluation tool in the future.Conclusion
Cerebral sodium MRI may have the potential to differentiate between different subgroups of migraine. This could lead to an enhanced and individualized therapy.Acknowledgements
No acknowledgement found.References
1. Harrington, Michael G et al. “Sodium MRI in a Rat Migraine Model and a NEURON Simulation Study Support a Role for Sodium in Migraine.” Cephalalgia?: an international journal of headache 31.12 (2011): 1254–1265. PMC. Web. 7 Nov. 2016.
2. Neumaier-Probst, E et al. “A double-tuned 1H/23Na resonator allows 1H-guided 23Na-MRI in ischemic stroke patients in one session.” Int J Stroke (2015), 10: 56–61. doi:10.1111/ijs.12547
3. Haneder, S et. al. “23Na-MRI of recurrent glioblastoma multiforme after intraoperative radiotherapy: technical note.” Neuroradiology (2015) 57: 321. doi:10.1007/s00234-014-1468-2
4. Eisele, P et. al “Heterogeneity of acute multiple sclerosis lesions on sodium (23Na) MRI.” Mult Scler July 2016 22: 1040-1047, first published on October 9, 2015 doi:10.1177/1352458515609430
5. Nagel, A. et. al. "Sodium MRI using a density-adapted 3D radial acquisition technique." Magn. Reson. Med. (2009), 62: 1565–1573. doi:10.1002/mrm.22157
Figures