1104
A "Glycolytic Index" for quantifying abnormal metabolism in human gliomas using multi-echo amine chemical exchange saturation transfer spin-and-gradient echo echoplanar imaging (ME-aCEST-SAGE-EPI) at 3T1University of California Los Angeles, Los Angeles, CA, United States, 2Radiological Sciences, University of California Los Angeles, Los Angeles, CA, United States
Synopsis
Abnormal metabolism is a hallmark of cancer. The current study demonstrates use of a novel imaging technique for fast, simultaneous pH- and hypoxia-weighted images using multi-echo amine chemical exchange saturation transfer spin-and-gradient-echo echoplanar imaging (ME-aCEST-SAGE-EPI). From these data, we demonstrate use of a “glycolytic index”, quantified as the ratio of relative acidity to metabolic rate of oxygen, in estimating metabolically active tumor tissue in 15 patients with gliomas. The glycolytic index showed unique heterogeneous metabolic contrast within the tumor region, and was able to easily stratify tumor from healthy tissue when compared with other imaging techniques.
Purpose
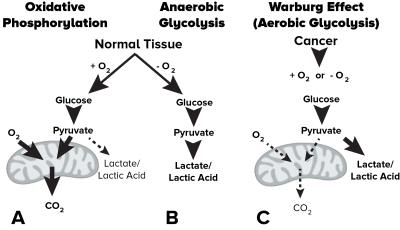
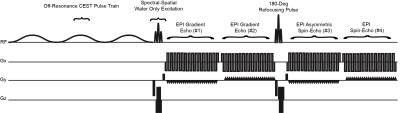
Abnormal metabolism is a hallmark of cancer. Notably, glycolysis is often enhanced in cancers even in the presence of abundant oxygen (i.e. the Warburg effect, Fig 1), resulting in increased interstitial acidosis from accumulating lactic acid. By labeling the fast exchanging amine protons on glutamine and other monoamines in high concentration within tumor tissues using targeted chemical exchange saturation transfer (CEST) imaging, we have demonstrated the resulting water MR image is sensitive to tissue pH within physiologic ranges1,2. In the current study we have designed and tested a simultaneous pH- and oxygen-sensitive molecular MRI technique using multi-echo amine chemical exchange saturation transfer spin-and-gradient echo echoplanar imaging (ME-aCEST-SAGE-EPI) in order to characterize the metabolic status of human gliomas in vivo in a clinically feasible scan time (Fig 2). This approach allows for concurrent estimation of interstitial pH, relative oxygen extraction fraction (rOEF), and relative cerebral metabolic rate of oxygen (rCMRO2). As proliferative tumor tissue is expected to be both acidic and hypoxic, we hypothesize that a “glycolytic index” defined as the ratio of pH-weighted image contrast to oxygenation may provide novel contrast in glioma tissues.Methods
The ME-aCEST-SAGE-EPI sequence was applied in phantoms of different glutamine concentrations and varying pH to verify the sensitivity of CEST contrast to acidity. Fifteen glioma patients then underwent ME-aCEST-SAGE-EPI using a pulse train of 3x100 ms, 6 μT saturation pulses with 29 spectral points centered around ±3 ppm and 0 ppm. An image with identical parameters but no saturation pulse (S0) was acquired for normalization. The readout consisted of two gradient echo (GRE) measurements at 14.0 and 34.1 ms, an asymmetric spin echo (aSE) measurement at 58.0 ms, and a spin echo (SE) measurement at 92.4 ms. Following B0 inhomogeneity correction, the magnetization transfer ratio asymmetry at 3.0 ppm was calculated for each voxel as MTRasym @ 3ppm = [S(-3ppm) – S(+3ppm)] / S0. Dynamic susceptibility contrast (DSC) perfusion MRI and anatomical images (T1-weighted post-contrast and FLAIR) were also acquired. The multi-echo readouts for S0 were used to estimate R2, R2* and R2’. Maps of relative cerebral blood flow (rCBF) and blood volume (rCBV) were estimated from perfusion data using in-house software3. Estimates of rOEF and rCMRO2 were calculated as rOEF = R2’/(c·rCBV) and rCMRO2 = (rCBF·R2’)/(c·rCBV), where c is a constant related to the field strength. A “glycolytic index” was created by quantifying the ratio of MTRasym at 3ppm to rCMRO2. All image data were registered to high resolution T1 post-contrast images and tumor regions of interest were defined by FLAIR hyperintensity and normal appearing white matter (NAWM).Results
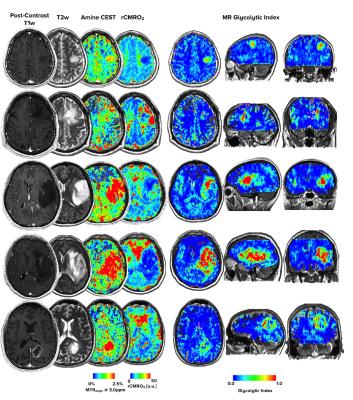
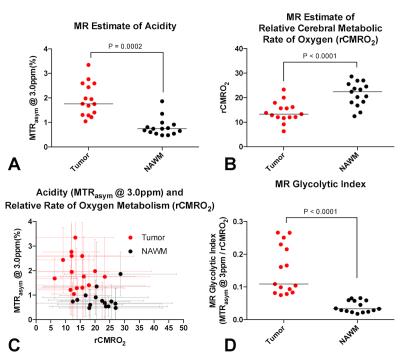
The ME-aCEST-SAGE-EPI sequence was verified to be sensitive to pH in glutamine phantoms, showing elevated MTRasym at 3ppm at low pH. In glioma patients, acidity was significantly elevated (Fig. 4A, P=0.0002) and rCMRO2 was significantly decreased (Fig. 4B, P<0.0001) in FLAIR hyperintense regions compared with NAWM. In many cases, anatomically homogeneous tumor exhibited extensive intratumoral metabolic heterogeneity. The glycolytic index provided improved stratification of tumor and NAWM compared with either acidity or rCMRO2 alone, with a specificity and sensitivity of 100% using a glycolytic index cutoff of 0.7 (Fig. 4C-D, P<0.0001). Importantly, the glycolytic index also provided unique contrast near the tumor boundaries that may be indicative of metabolically active tumor undergoing high rates of glycolysis (Fig. 3).Discussion
Aerobic glycolysis is a trademark of cancer metabolism. Anatomic imaging fails to provide important information about tumor metabolism, which is highly heterogeneous. Combining oxygenation and pH-weighted measurements provides a novel contrast that can be used for identification of the most metabolically abnormal tumor regions and may be useful for differentiating treatment effects from growing tumor. Further research is warranted to determine whether biopsy results show elevated histologic proliferation in regions of elevated glycolytic index.Conclusion
Simultaneous pH- and hypoxia-weighted metabolic MRI provides unique insight into the glycolytic state in human gliomas.Acknowledgements
No acknowledgement found.References
1Harris RJ, Cloughesy TF, Liau LM, Prins RM, Antonios JP, Li D, Yong WH, Pope WB, Lai A, Nghiemphu PL, Ellingson BM. pH-weighted molecular imaging of gliomas using amine chemical exchange saturation transfer MRI. Neuro Oncol 2015 Nov;17(11):1514-24
2 Harris RJ, Cloughesy TF, Liau LM, Nghiemphu PL, Lai A, Pope WB, Ellingson BM. Simulation, phantom validation, and clinical evaluation of fast pH-weighted molecular imaging using amine chemical exchange saturation transfer echo planar imaging (CEST-EPI) in glioma at 3 T. NMR Biomed. 2016 Nov;29(11):1563-1576.
3 Leu K, Boxerman JL, Cloughesy TF, Lai A, Nghiemphu PL, Liau LM, Pope WB, Ellingson BM. Improved Leakage Correction for Single-Echo Dynamic Susceptibility Contrast Perfusion MRI Estimates of Relative Cerebral Blood Volume in High-Grade Gliomas by Accounting for Bidirectional Contrast Agent Exchange. AJNR Am J Neuroradiol. 2016 Aug;37(8):1440-6.
Figures