1102
Glycine, a marker of survival in paediatric brain tumours measured with non-invasive Magnetic Resonance Spectroscopy: A five-year survival analysis.1University of Birmingham, Birmingham, United Kingdom, 2Birmingham University Imaging Centre, University of Birmingham, Birmingham, United Kingdom, 3Birmingham Children's Hospital NHS Foundation Trust, Birmingham, United Kingdom, 4Imaging & Medical Physics, University Hospitals Birmingham NHS Foundation Trust, Birmingham, United Kingdom
Synopsis
Brain tumours have a high mortality rate and are the most common solid tumour of childhood. MRS is a non-invasive imaging technique that measures tumour metabolites which can provide prognostic information to aid clinical management. The metabolite glycine is associated with proliferation and tumorigenesis through the one-carbon metabolic cycle. Glycine concentration has been shown to increase with tumour grade using short echo time MRS at 1.5T, however has not been assessed as a survival marker. This study investigates glycine as a marker of survival in paediatric brain tumours and assesses its added value compared to other established metabolite survival markers.
Purpose
Brain tumours have a high mortality rate and are the most common solid tumour of childhood1. Identification of high risk patients may allow for better treatment stratification and more effective disease management, increasing survival and reducing treatment related damage. Magnetic Resonance Spectroscopy (MRS) provides a non-invasive measure of brain tumour metabolism and identifies possible survival markers to aid in clinical management of these patients. Glycine, the smallest amino acid, is an inhibitory neurotransmitter and has recently been linked to cancer cell proliferation and tumorigenesis, through the one-carbon metabolism cycle2,3. MRS studies have shown that glycine is a potential marker of tumour grade in adult and paediatric brain tumours4,5,6. Previous reports have shown that glycine concentrations are particularly elevated in medulloblastomas5 and glioblastomas7. Ex-vivo analysis has also demonstrated higher concentrations in high-grade brain tumours8. To date, glycine measured by MRS has not been investigated as a potential survival marker in paediatric brain tumours. The aims of this study were to investigate glycine as a marker of five year survival and its added value in determining the prognosis prediction potential of MRS in paediatric brain tumours within a clinical setting.
Methodology
One hundred and twenty children with newly diagnosed brain tumours were examined with MRS prior to treatment from 2003-2010, with five year follow up. Histopathologically confirmed diagnoses included; piloctytic astrocytoma, medulloblastoma, ependymoma, OPG, ganglioma, DNET, pilomyxoid astrocytoma, ependymoma, astrocytoma, diffuse astrocytoma, DIPG, anaplastic ependymoma, ATRT, pineoblastoma, TPG, glioblastoma and SPNET tumours. Clinical data was obtained including gender, age at diagnosis, and dates of death. Tumour diagnosis was confirmed by a multidisciplinary team using histopathological, clinical and radiological information. Most patients were treated with surgical resection, followed by fractionated radiotherapy and chemotherapy with the protocol based on diagnosis and clinical risk factors.
MR imaging and MRS were acquired on a 1.5T Siemens Symphony Magnetom. Single voxel spectroscopy PRESS was used (TR = 1500ms, TE = 30ms, Nx = 126 or 256). The MRS voxel was placed within the solid component of the primary tumour. Unprocessed MRS signals were analyzed using TARQUIN 9 software (version 4.3.6). Spectra were excluded based on displaced voxel location, unstable spectral baseline, presence of artefacts, signal to noise ratio (SNR) < 4, overall metabolite linewidths > 0.15ppm and water linewidth >10Hz.
Survival analysis was performed on individual metabolites using Kaplan-Meier, log-rank tests and hazard ratios, based on median concentration cut-off values. Individual metabolite markers of prognosis were combined to assess the prognosis prediction potential of MRS. The added value of glycine to the MRS prognosis prediction potential was assessed with and without glycine in the analysis. Statistical tests were performed in the R using the survival library where P<0.05 was deemed significant.
Results and Discussion
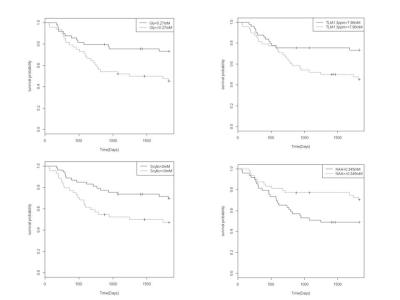
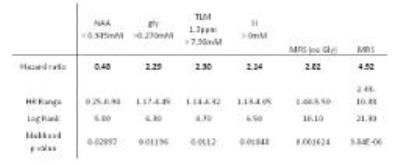
The final cohort consisted of 97 patients after quality control criteria were applied. All 19 patient deaths were a direct result of their disease. The mean±SD age of the patient cohort was 7.51±5.4 years and 71% were male, however these factors were not found to influence survival. Glycine (gly), total lipids and macromolecules (TLM) at 1.3ppm, scyllo-inositol (SI) and N-acetylaspartate (NAA) were found to be biomarkers of prognosis Figure 1. NAA, TLM at 1.3ppm and SI have been previously reported as biomarkers in the literature 10.
The detection of glycine is potentially hindered in spectra due to the spectral overlap with Myo-inositol at 3.55ppm and 3.6ppm, respectively. However, a pediatric brain tumour study reported a correlation between in-vivo and ex-vivo measures of glycine and additionally between short and long echo times 5,10. This finding provides certainty that glycine can be measured in short echo 1.5T MRS in paediatric brain tumours (Figure 2).
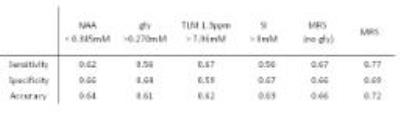
The prediction of survival using glycine was comparable to other individual metabolite survival markers in the log rank, hazard ratio and accuracy analyses, Table 1 and 2. The survival prediction potential of MRS assessment showed that the predictive potential was larger with glycine included, Table 1, Figure 3. The accuracy of MRS survival prediction of death within 5 years increased with the inclusion of glycine. Glycine concentration levels therefore provide an additional marker to aid survival prediction using MRS.
Conclusions
Glycine was found to be a survival marker using MRS in paediatric brain tumours. This is an important finding aligns with the recent finding of glycine being vital to cancer cell proliferation in tumours. The addition of glycine to other proven metabolite survival markers improves the prognosis prediction potential of MRS, and thus increases the confidence in MRS as a tool for aiding in the clinical management of brain tumour patients.
Acknowledgements
We would like to thank the staff of the Radiology Department at Birmingham Children’s hospital for their help in collecting the MRS data. We would also like to thank Jane Crouch for coordinating the study, Rachel Grazier, Heather Rose for data management and Cay Shakespeare for patient and parent liaison.
References
1.Bleyer W et al. 8 drugs in 1 day chemotherapy for brain tumors: a new approach and rationale for preradiation chemotherapy. Med Pediat Oncol: 1983 11: 213
2. Locasale W. Serine, glycine and the one-carbon cycle: cancer metabolism in full circle. Nat Rev Cancer. 2013; 13(8): 572–583.
3. Amelio I et al. Serine and glycine metabolism in cancer. Trends in Biochemical Sciences. 2014;39(4):191-198
4. Kinoshita Y, Yokota A. Absolute concentrations of metabolites in human brain tumors using in vitro proton magnetic resonance spectroscopy. NMR Biomed. 1997; 10: 2–12
5. Davies NP et al. Non-invasive detection of glycine as a biomarker of malignancy in childhood brain tumours using in-vivo 1H MRS at 1.5 tesla confirmed by ex-vivo high-resolution magic-angle spinning NMR. NMR Biomed. 2010; 23:80–87.
6. Carpinelli G, Carapella CM, Palombi L, Raus L, Caroli F, Podo F.Differentiation of glioblastoma multiforme from astrocytomas by invitro 1H MRS analysis of human brain tumors. Anticancer Res. 1996;16: 1559–1563.
7. Righi V et al. High-resolution magic angle spinning magnetic resonance spectroscopy detects glycine as a biomarker in brain tumors. International Journal of oncology.2010:36;301-306.
8. Carapella CM, Carpinelli G, Knijn A, Raus L, Caroli F, Podo F. Potential role of in vitro 1H magnetic resonance spectroscopy in the definition of malignancy grading of human neuroepithelial brain tumours. Acta Neurochir Suppl. 1997; 68: 127–132.
9. Wilson M. et al. A constrained least-squares approach to the automated quantitation of in vivo ¹H magnetic resonance spectroscopy data. Mag Reson Med 2011;65:1-12
10. Hattingen E, Lanfermann H, Quick J, Franz K, Zanella FE, Pilatus U. 1H MR spectroscopic imaging with short and long echo time to discriminate glycine in glial tumours. MAGMA. 2009; 22:33–41.
Figures