1101
Towards opto-fMRS: Ultra high field MRS measurement of T2* changes due to optogenetic stimulation1Department of Psychiatry, McGill University, Montreal, QC, Canada, 2Centre d'Imagerie Cérébrale, Douglas Mental Health University Institute, Montreal, QC, Canada, 3Integrated Program in Neuroscience, McGill University, Montreal, QC, Canada, 4Biomedical Engineering, McGill University, Montreal, QC, Canada, 5Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom
Synopsis
Combining optogenetics with fMRI/fMRS (opto-fMRI/opto-fMRS) offers the unique potential to study the whole brain functional or neurochemical effects of stimulating specific neuronal populations within a given brain region. In this study, we investigated the BOLD functional changes and neurochemical changes resulting from optogenetic stimulation of glutamatergic or GABAergic neurons in the medial septum. Stimulation of both glutamatergic and GABAergic neurons in the medial septum resulted in prominent bold activation within the hippocampus, and in other regions. This study represents a unique imaging investigation into the functional response to stimulation of multiple distinct neuronal populations within a single brain region.
Purpose
Hippocampal theta rhythms play an important role in learning, spatial navigation, and memory formation. Recent work using optogenetics has shown that activation of glutamatergic or GABAergic neuronal populations in the medial septum drives hippocampal theta rhythms [1,2]. The purpose of this study is to investigate: 1) the hippocampal BOLD functional response and 2) the hippocampal neurochemical response to optogenetic stimulation of glutamatergic or GABAergic neurons in the medial septum.Methods
The medial septum provides important connections to the hippocampus and plays a essential role in theta rhythm generation. Recently, optogenetic activation of GABAergic and glutamatergic populations in the medial septum has been shown to drive theta oscillations (6-10 Hz) in the hippocampus [1,2]. To separately investigate the effects of stimulating septal GABAergic or glutamatergic neuronal populations on hippocampal function and neurochemistry, four VGlut2-Cre knock-in homozygote mice and four VGAT-ires-Cre knock-in homozygote mice were prepared for optogenetics experimentation. All experiments were performed according to protocols approved by McGill University’s animal research committee and were in accordance with the guidelines put forth by the Canadian Council on Animal Care. All mice were injected in the medial septum with a Cre-dependent adeno-associated viral vector (AAVdj-Efla-DIO-hChR2(E123T/T159C)-eYFP) to trigger expression of the ChETATC excitatory opsin. Three weeks following viral transfection, an optic fiber (Thorlabs) connected to a ferrule (Precision Fiber Products), was implanted at a 5° angle to target slightly above the medial septum (AP 0.86, ML -0.2, DV-3.83). One week following implantation, mice were anesthetized with 1-2% isoflurane, and scanned in 7 Tesla Bruker (Billerica, MA, USA) BioSpec 70/30 scanner using a home-built, single-loop receive-only surface coil and a vendor provided volumetric transmit coil. An anatomical RARE image (TR = 2585 ms, echo spacing = 12 ms, RARE factor = 8, effective TE = 48 ms, slice thickness = 1 mm, FOV = 15 x 15 mm, matrix = 256 x 256, 4 averages) was acquired to visualise fiber positioning and to guide placement of a 1.5 x 3.3 x 3 mm voxel in right or left dorsal hippocampus. Following FASTMAP shimming [3] in the hippocamus, localized MRS data were acquired using the PRESS sequence during optogenetic stimulation of the medial septum using a blue (473 nm) laser (LaserGlow Technologies, Toronto, Canada). Optogenetic stimulation consisted of a 10 Hz pulse train with a 50% duty cycle, applied in a one-minute off, one-minute on block design for a total of eight minutes. For assessment of hippocampal BOLD effects during optogenetic stimulation, PRESS spectra (TR/TE=3000/25 ms, spectral width = 4006 Hz, 2048 points, 160 averages) were acquired without water suppression. As a control condition, an identical water unsuppressed PRESS dataset was acquired without optogenetic stimulation. For assessment of hippocampal neurochemical effects during optogenetic stimulation, PRESS spectra were acquired as above, but with TE=12 ms and VAPOR water suppression. The BOLD signal timecourse was obtained from the water unsuppressed PRESS data by log-transforming the time domain signal for each average and performing a linear fit to determine the slope, as a measure of the T2* decay rate. Finally, whole-brain BOLD effects were assessed in one VGAT-Cre mouse using a gradient echo EPI sequence (TR/TE=2000/15 ms, slice thickness = 0.8 mm, FOV = 17.6 x 14.8 mm, matrix = 60 x 48, 240 volumes) and the same stimulation paradigm as above. BOLD EPI functional data were analysed using FSL Feat (FMRIB Centre, Oxford, UK) and registered to the corresponding individual’s anatomical image using ANTs (PICSL, PA USA).Results
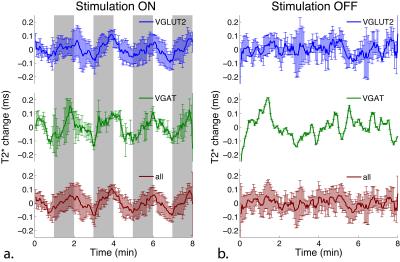
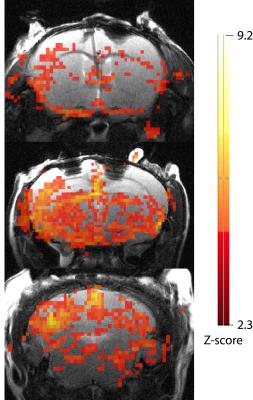
Seven mice successfully underwent MR scans. Figure 1a shows the hippocampal BOLD signal timecourse, averaged across all mice, VGlut2-Cre mice only, and VGAT-Cre mice only. The signal timecourse shows clear hippocampal BOLD activation in response to optogenetic stimulation of both glutamatergic (VGlut2) and GABAergic (VGAT) cell populations in the medial septum. Figure 1b shows the signal timecourses when no stimulation was applied. As expected, no activation was observed in the no-stimulation condition. Figure 2 shows the BOLD signal activation map from an individual VGAT-Cre mouse, overlayed with the anatomical image. Neurochemical analyses from water suppressed MRS data are currently in progress.Discussion and Conclusions
Optogenetic stimulation of septal glutamatergic and GABAergic neurons in mice produced strong (1-2%) BOLD signal activations both in the hippocampus and elsewhere in the brain. Slightly stronger BOLD activation was observed from stimulation of GABAergic cells, as compared to glutamatergic cells. Analysis of neurochemical changes in response to optogenetics stimulation is currently in progress. This study represents a novel imaging investigation into the functional and neurochemical response to stimulation of multiple distinct neuronal populations within a single brain region.Acknowledgements
This research was funded by the Oxford-McGill-ZNZ Tripartite Partnership in the Neurosciences, awarded to JN and UE, and an FRSQ Subvention pour projet de développement stratégique innovants 2012-2016 (Grant No. 26679) awarded to MR.References
1. Robinson J et al. J Neurosci 2016;36(10):3016-23. 2. Bender F et al. Nat Commun 2015;6:8521. 3. Gruetter et al. Magn Reson Med 1992; 29:804-11.
Figures