1096
Accelerated Diffusion-Sensitized MR Imaging of the Eye and Orbit at 3.0 T and 7.0 T free of Geometric Distortions Using a Combined RARE-EPI Acquisition Technique1Berlin Ultrahigh Field Facility (B.U.F.F.), Max Delbrueck Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany, 2MRI.TOOLS GmbH, Berlin, Germany, 3Department of Ophthalmology, University of Rostock, Rostock, Germany, 4Experimental and Clinical Research Center (ECRC), a joint cooperation between the Charité Medical Faculty and the Max Delbrueck Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany
Synopsis
Diffusion-weighted imaging of the eye and orbit is an emerging MRI application to provide guidance during diagnostic assessment and treatment of ophthalmological diseases. RARE based diffusion-sensitized imaging (ms-RARE) provides images free of geometric distortions. Yet imaging speed, RF power deposition and artifacts by involuntary eye motion remain a concern. Combined acquisition techniques (CAT) merging RARE and EPI within one echo train offer the possibility to shorten acquisition times and relax specific absorption rate constraints. This study examines the applicability of RARE-EPI CAT for diffusion-sensitized imaging of the eye and orbit free of geometric distortions at 3.0 T and 7.0 T.
Purpose
MRI of the spatial arrangements of the eye segments and their masses is an emerging application increasingly used in (pre-)clinical imaging and diagnostic radiology1-5. Diffusion weighted MRI (DWI) probes self-diffusion of water in tissue on a microscopic level and holds the promise to enhance the diagnostic accuracy over anatomic ophthalmic imaging6. Diffusion-sensitized segmented split-echo rapid acquisition with relaxation enhancement imaging (RARE) provides high-spatial resolution images of the eye, orbit and nervus opticus at 3.0T and 7.0T7. Geometric distortions that are observed for EPI-DWI approaches are offset by DWI using RARE, but radiofrequency (RF) power deposition, scan time constraints and sensitivity to involuntary eye motion remain a legitimate concern. Previously proposed combined acquisition techniques (CAT)8 merging RARE and EPI9,10 within one acquisition hold the promise to maintain the anatomic fidelity of RARE based diffusion-weighted imaging while simultaneously relaxing specific absorption rate (SAR) and imaging speed constraints. This study examines the applicability of diffusion-sensitized RARE-EPI CAT for ophthalmic imaging.Methods
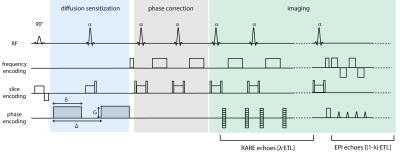
Conventional RARE11 was modified (according to 8) by replacing [(1-λ)×ETL] RARE echoes by EPI echoes (Figure 1, ETL=echo train length). The factor λ (0≤λ≤1) defines the fraction of echoes within the echo train covered by the RARE module. Data were acquired center-out in k-space together with a minimal TE for diffusion-weighted acquisitions while a linear phase encoding scheme in conjunction with partial Fourier sampling was applied for T2-weighted images. Split echo acquisition and 1D navigator based phase correction were incorporated as illustrated in Figure 1 to facilitate robust diffusion sensitization (details in 7). To examine the geometric fidelity of RARE-EPI CAT, FLASH (anatomic reference), traditional RARE, single-shot-EPI (ss-EPI) and readout-segmented-EPI (rs-EPI)12,13 phantom imaging was performed at 3.0T (Verio, Siemens, Erlangen, Germany). Quantification of geometric distortion was performed using center of gravity analysis. In-vivo anatomical brain images were acquired to demonstrate the comparability of RARE-EPI CAT to conventional RARE images. To elucidate the propensity of diffusion-sensitized RARE-EPI CAT, ss-EPI and rs-EPI to geometric distortions ADC mapping of the eyes was conducted at 3.0T. To demonstrate the applicability of RARE-EPI CAT at ultrahigh magnetic field strengths ADC mapping of the eyes was performed at 7.0T (Magnetom, Siemens, Erlangen, Germany) using RARE and RARE-EPI CAT. A dedicated six-element transceiver coil array consisting of loop elements was employed at 7.0T5 (MRI.TOOLS GmbH, Berlin, Germany).Results
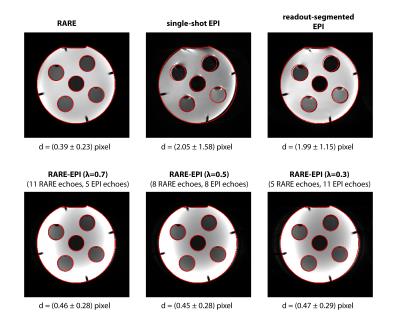
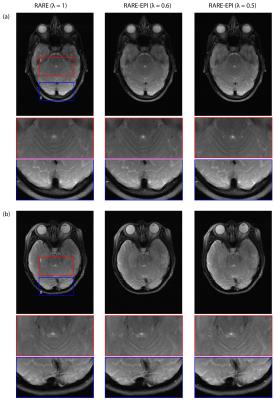
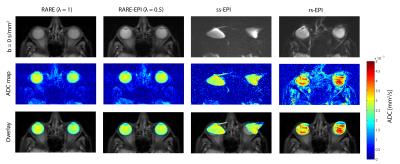
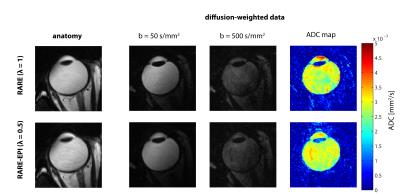
For RARE minor distortions were observed in the phantom images with respect to the FLASH reference that can be attributed to differences in the point spread function of the two pulse sequences (Figure 2). The deviations were severely increased for ss-EPI and rs-EPI. Using a factor λ=0.5 for RARE-EPI CAT increased the mean displacement by 15% versus the value obtained for traditional RARE. For ETL=16 plus 3 dummy pulses the administered RF power deposition is reduced by 36% for λ=0.5 based on calculations using the integral of the RF pulses as a measure. Figure 3 shows anatomical data of two exemplary volunteers for brain slices including the eyes comparing conventional RARE with RARE-EPI CAT. Image quality and contrast are maintained for RARE-EPI CAT which manifests itself in the visibility of subtle anatomical structures as demonstrated in the zoomed views of Figure 3. To illustrate the immunity of diffusion-sensitized RARE-EPI CAT to geometric distortions in comparison to RARE, ss-EPI and rs-EPI, ADC maps acquired at 3.0T are presented in Figure 4. Severe geometric distortions were observed for ss-EPI while rs-EPI showed moderate distortions. RARE-EPI CAT exhibits anatomical integrity comparable to RARE based ADC mapping. No distortions and no other imaging artifacts were detected as indicated by the match between the ADC maps superimposed to reference images. Figure 5 demonstrates the applicability of diffusion-sensitized RARE-EPI CAT at 7.0T which is underscored by the qualitative match between the ADC maps acquired using RARE and RARE-EPI CAT.Discussion
Our data demonstrate that RARE-EPI CAT meets the requirement of high anatomic fidelity and enables diffusion-weighted imaging of the eye and orbit free of geometric distortion. This offers means to accelerate data acquisition by increasing the number of slices or lowering TR and helps to reduce the propensity to involuntary eye motion. The relaxed SAR constraints of combined RARE-EPI CAT facilitates the incorporation of multiband RF pulses14 into the pulse sequence to further enhance anatomic coverage.Conclusion
This study showed that
diffusion-sensitized RARE-EPI CAT provides images of the eye and orbit free of
geometric distortions. The results underline the challenges of ocular EPI at 3.0T and 7.0T and demonstrate that these issues can be offset by using a combined
RARE-EPI CAT imaging technique.Acknowledgements
No acknowledgement found.References
1. Mafee MF, et al. Neuroimag Clin N Am. 2005; 15(1):23
2. Apushkin MA, et al. Neuroimag Clin N Am. 2005; 15(1):49.
3. Sepahdari AR, et al. Am J Neuroradiol. 2012; 33(2):314.
4. Beenakker JWM, et al. NMR Biomed. 2013; 26(12):1864.
5. Graessl A, et al. Invest Radiol. 2014; 49(5):260.
6. Norris DG, et al. NMR Biomed. 1994; 7(7):304.
7. Paul K, et al. Invest Radiol. 2015; 50(5):309.
8. Hillenbrand C, et al. MAGMA. 2000; 10(3):183.
9. Jakob PM, et al. Magn Reson Med. 2002; 47(3):425.
10. Choli M, et al. MAGMA. 2013; 26(4):411.
11. Hennig J, et al. Magn Reson Med. 1986; 3(6):823.
12. Porter DA, et al. Magn Reson Med. 2009; 62(2):468.
13. Heidemann RM, et al. Magn Reson Med. 2010; 64(1):9.
14. Barth M, et al. Magn Reson Med. 2016; 75(1):63.
15. Williams CFM, et al. Magn Reson Med. 1999; 41(4):734.
Figures