1081
A multivariate machine learning framework for psychosis: integrating diffusion and structural MRI1Psychosis Studies, King's College London, London, United Kingdom, 2Centre for the Developing Brain, Division of Imaging Sciences and Biomedical Engineering, King's College London
Synopsis
Machine learning is increasingly being used in psychiatric research for patient classification and stratification. Current machine learning approaches are largely univariate with main focus on structural MRI and do not account for the significant white matter connectivity alterations associated with the disorder. With the steady growth of multi-centre multi-modal neuroimaging studies there is a need for multivariate machine learning frameworks that can integrate these different types of information. Here, we propose an automated multivariate machine learning pipeline to integrate state-of-the-art structural and diffusion features, based on well-established widely-available software packages to keep implementation and replication as simple as possible.
INTRODUCTION
Previous studies have shown that it is possible to distinguish psychosis patients from healthy controls using either structural or diffusion MRI. However, structural images report on different abnormalities from diffusion images, and combining these two sources of complementary information would therefore be expected to be beneficial. In addition, previous studies have either used voxel intensities from T1-weighted images as input features to machine learning algorithms, or fractional anisotropy or mean diffusivity from diffusion images. However, these measures are very high dimensionality, making it difficult to identify patterns that relate specifically to alterations found in psychosis. Measures such as cortical thickness, mean curvature, volume, surface area and connectivity can provide low dimensional, highly informative features that can be combined into a multivariate framework, which may be able to account simultaneously for most structural and connectivity changes found in psychosis. In this study, we propose to use state-of-the-art structural and diffusion analysis methods to extract features likely to be sensitive to a range of psychiatric and neurological disorders. Including connectivity features is particularly interesting given the growing literature supporting a role for white matter alteration in psychosis. METHODS
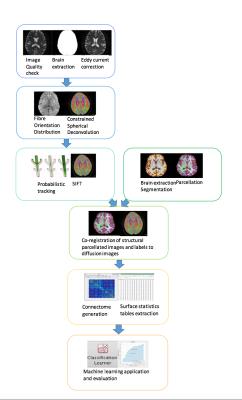
The data used here was made available through the COBRE consortium1, consisting of 105 participants (65 controls, 40 schizophrenia) imaged in a Siemens 3T Trio TIM scanner using 12-channel head coils. Structural MRI data consist of high-resolution T1-weighted MPRAGE images (TR: 2530ms, TI: 900ms, flip angle: 7°, FOV: 256×256mm, slab thickness: 176mm, 1mm isotropic resolution). Diffusion MRI data were acquired using a pulsed-gradient spin-echo EPI sequence (TR: 9000ms, TE: 84ms, FOV: 256×256mm, 2mm isotropic resolution, 35 gradient directions, b=800s/mm2, 5 b=0 volumes). A graphical overview of our proposed pipeline is shown in Figure 1. First, diffusion data were pre-processed using FSL2 and MRtrix33. Data were corrected for motion and eddy-current distortions4, and analysed using constrained spherical deconvolution5 to obtains maps of the fibre orientation distribution (FOD). These were used for probabilistic tractography6 producing 100 million streamlines over the whole brain, which were subsequently filtered down to 10 million using SIFT7 to provide streamlines counts representative of white matter connectivity8.Analysis of the structural images was performed using Freesurfer 5.3.09, including intensity normalization, brain extraction, segmentation, parcellation, pial surface and white matter mask creation. All extracted brain masks and parcellation were overlaid on the anatomical images and their quality was checked across all slices and planes. Measures of area, volume, thickness, curvind, foldind and meancurv were extracted for each ROI according to the Desikan-Killiany atlas. The parcellation images were co-registered to the diffusion data using FLIRT10, and the matrix of region-to-region connectivity (the connectome) was computed as the number of post-SIFT streamlines connecting the regions. After registration all surface statistics values were extracted for each subject and concatenated into a table of subjects×features, and similarly for the region-to-region connectivity values. Machine learning was performed on these matrices, as well as on the full concatenated structural and connectivity matrix (Figure 2). All Machine learning was performed using the matlab classification learner application11 with 5-fold Leave One Out Cross Validation using Support Vector Machines.RESULTS
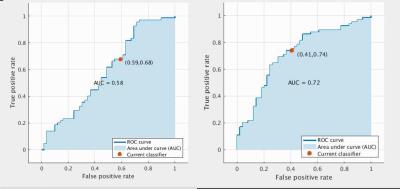
Classification accuracy of 56.1% was achieved using the structural features only (AUC=0.58), compared to 64.9% using the connectivity features only (AUC=0.64). Using the full combined structural and connectivity feature matrix, 67.5% accuracy was achieved (AUC=0.72) (Figure 3).
DISCUSSION AND CONCLUSION
In this work we propose a fully automated and reproducible pipeline to integrate structural and diffusion data for classification of psychosis patients from healthy controls. We observed a considerable increase in classification accuracy when including the connectivity information, supporting the notion that disturbances in white matter connectivity are implicated in psychosis. To our knowledge, this is the first model integrating diffusion and structural data within a machine learning framework in psychosis. We anticipate that this type of disease-driven, automated multi-modal framework combined with large-scale multi-modal studies will be beneficial for identification of meaningful biomarkers, prognosis, stratification and prediction of treatment response in psychiatric research.Acknowledgements
Vasiliki Chatzi is supported by a Departmental scholarshipReferences
1. http://coins.mrn.org
2. http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/
3. http://www.mrtrix.org/
4. Andersson JLR, Sotiropoulos SN (2016) An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage 125:1063–1078.
5. Tournier JD, Calamante F, Connelly A (2007) Robust determination of the fibre orientation distribution in diffusion MRI: Non-negativity constrained super-resolved spherical deconvolution. NeuroImage 35:1459–1472. doi: 10.1016/j.neuroimage.2007.02.016
6. Tournier J-D, Calamante F, Connelly A (2010) Improved Probabilistic Streamlines Tractography by 2nd Order Integration Over Fibre Orientation Distributions. In: Proceedings of the International Society for Magnetic Resonance in Medicine. Stockholm, Sweden, p 1670Smith RE,
7. Tournier J-D, Calamante F, Connelly A (2013) SIFT: Spherical-deconvolution informed filtering of tractograms. Neuroimage 67:298–312. doi: 10.1016/j.neuroimage.2012.11.049
8. Smith RE, Tournier J-D, Calamante F, Connelly A (2015) The effects of SIFT on the reproducibility and biological accuracy of the structural connectome. NeuroImage 104:253–265. doi: 10.1016/j.neuroimage.2014.10.004
9. http://surfer.nmr.mgh.harvard.edu
10. Jenkinson, M., Bannister, P., Brady, J. M. and Smith, S. M. Improved Optimisation for the Robust and Accurate Linear Registration and Motion Correction of Brain Images. NeuroImage, 17(2), 825-841, 2002.
11. https://uk.mathworks.com/help/stats/classificationlearner-app.html
Figures