1079
Reduced Functional Network Segregation is Associated with Reduced Structural Network Integration and Cost Pre-Operatively in Neonates with Complex Congenital Heart Disease (CHD)1Radiology, Children's Hospital of Pittsburgh of UPMC, Pittsburgh, PA, United States, 2Division of Cardiology, Children's Hospital Los Angeles, 3Division of Pediatric Cardiothoracic Surgery, Children's Hospital Los Angeles, 4Department of Biomedical Informatics, University of Pittsburgh, 5Radiology, Children's Hospital Los Angeles, 6Division of Pediatric Cardiology, University of Pittsburgh School of Medicine, 7Department of Critical Care, University of Pittsburgh School of Medicine, 8Division of Neonatology, Children's Hospital Los Angeles
Synopsis
Neonates with complex congenital heart disease (CHD) pre-operatively show alterations in both structural network topology (as assessed via DTI) and functional network topology (as assessed via rs-fcMRI). Structurally, decreases in global efficiency, transitivity, and nodal efficiency are driven by decreased network cost and reflect alterations in white matter microstructure such as reduced fiber density. Functionally, CHD neonates display decreased network segregation in the later-developing frontal and temporal lobes, independent of cost, which likely reflect alterations at a more hierarchical level of architecture. These results may stem from different etiologies of brain dysmaturation (hypoperfusion vs. genetic factors).
Purpose
Complex congenital heart disease (CHD) is associated with brain dysmaturation in the fetal and neonatal period, but the associated functional and structural network correlates of this aberrant brain maturation in the pre-operative period is unknown. We applied both diffusion imaging tensor (DTI) and resting-state functional connectivity MRI (rs-fcMRI) and graph analysis to neonatal CHD patients pre-operatively, as well as normal healthy controls.Materials and Methods
Participants: Complex CHD and healthy term neonates were prospectively recruited from Children’s Hospital Los Angeles (CHLA) and Children’s Hospital of Pittsburgh (CHP) from 2009-2016. Specific CHD pathologies included single ventricle physiology, aortic arch obstruction, conotruncal, aortic arch obstruction, aortic valve outflow obstruction, and heterotaxy.
Acquisition: At CHP, participants were scanned either on a GE 1.5 T (DTI only), GE 3 T, or Siemens Skyra 3T system; at CHLA, participants were scanned on a Philips 3T Achieva system, using a multi-channel coil. DTI acquisition parameters were: b = 700 s/mm2, at least 30 acquired diffusion-weighted images. Rs-fcMRI acquisition parameters were: TR = 2 s, TE ≈ 35 ms, total scan time five minutes.
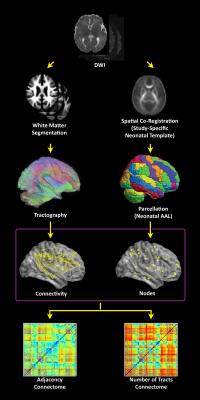
DTI preprocessing (Figure 1): Motion and eddy current artifacts were corrected using routines in FSL (FMRIB, Oxford, UK). The parcellation atlas1 was back-transformed into native space using a study-specific FA template. A white matter segmentation (independent of absolute FA values), was performed using the FA map, neonatal templates1 and the spm_preproc8 routine in SPM8. Deterministic tractography in native space was carried out using routines in IDL. Streamlines were constructed starting from each voxel with WM probability > 0.78 and were continued in both directions with stopping criteria of turning angle > 45 degrees or WM probability < 0.78. Adjacency and number of tracts connectomes were computed.
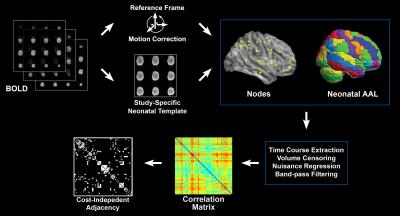
Rs-fcMRI preprocessing (Figure 2): After spatial normalization to the neonatal template and time course extraction from the parcellation atlas1, previously validated methods2,3 were used to minimize possible spurious findings due to participant motion, including volume censoring according to framewise displacement (FD) > 0.2 mm or intensity-related (DVARS) > 25, regressing out of nuisance parameters including global signal, motion parameters, and linear and quadratic drift; and band-pass filtering (0.009 Hz < f < 0.08 Hz). Correlation matrices were constructed as the absolute value of the correlation coefficient between two time courses. Binary unweighted graphs were obtained via thresholding at cost values ranging from 0.05 to 0.45, step 0.05.
Graph analysis: Graph metrics were computed using routines in Brain Connectivity Toolbox (Indiana University, Bloomington, IN) and IDL. For DTI, global and nodal metrics were used as dependent variables in a standard GLM. For rs-fcMRI, both global and regionally-averaged (by lobe) nodal metrics were used as dependent variables in a mixed-effects GLM (due to the multiple cost values). CHD status was the covariate of interest, with scanner, sex, post-conceptional age (PCA) at birth and at scan, as covariates of no interest; for DTI, analyses were repeated with cost included as a covariate.
Results
Participants: For DTI, data was successfully collected from 96 controls and 70 CHD neonates; for rs-fcMRI, from 75 controls and 27 CHD neonates.
DTI: Globally, CHD patients scanned pre-operatively displayed significantly reduced cost, global efficiency, and transitivity (p < 0.001), and a wide region of reduced nodal efficiency (Figure 3), for both adjacency and number of tracts connectomes. These differences are driven by the reduced cost, as they did not remain significant after controlling for cost.
Rs-fcMRI: Globally, CHD patients displayed reduced transitivity and modularity (p < 0.05), indicating decreased network segregation. Regionally, these differences are driven by decreased nodal segregation (as reflected by decreased local efficiency) in the frontal and temporal lobes (p < 0.01) and decreased regional segregation (as reflected by increased participation coefficient) in the occipital lobe (p < 0.05).
Discussion
Structurally, CHD patients pre-operatively display microstructural white matter differences (reduced fiber density). The etiology is likely related to the last trimester, where reduced fetal brain volume, neuronal-axonal metabolism and oxidative metabolism have been shown4,5. These differences in structural network topology may be distinct from differences in functional topology, which also reflect brain dysmaturation but are regionally distinct. Later-developing regions (frontal and temporal lobes) display reduced functional segregation.
Differences between differences in structural and functional topologies may provide useful biomarkers to distinguish in utero etiologies (hypoperfusion vs. genetic factors) which may drive neurodevelopmental outcomes in CHD patients6,7. Additionally, future research with post-operative CHD patients may investigate the effects of surgery on brain topology.
Conclusion
Preoperative brain dysmaturation in neonates with complex
CHD is characterized by functional network segregation associated with reduced
structural network integration driven by cost. Acknowledgements
No acknowledgement found.References
1. Shi F, Yap PT, Wu G, Jia H, Gilmore JH, Lin W, et al. Infant brain atlases from neonates to 1- and 2-year-olds. PloS one. 2011;6(4):e18746.
2. Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage. 2014;84:320-41.
3. Power JD, Schlaggar BL, Petersen SE. Recent progress and outstanding issues in motion correction in resting state fMRI. NeuroImage. 2015;105:536-51.
4. Limperopoulos C, Majnemer A, Shevell MI, Rosenblatt B, Rohlicek C, Tchervenkov C. Neurodevelopmental status of newborns and infants with congenital heart defects before and after open heart surgery. The Journal of pediatrics. 2000;137(5):638-45.
5. Sun L, Macgowan CK, Sled JG, Yoo SJ, Manlhiot C, Porayette P, et al. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation. 2015;131(15):1313-23.
6. Mussatto KA, Hoffmann RG, Hoffman GM, Tweddell JS, Bear L, Cao Y, et al. Risk and prevalence of developmental delay in young children with congenital heart disease. Pediatrics. 2014;133(3):e570-7.
7. Donofrio MT, Duplessis AJ, Limperopoulos C. Impact of congenital heart disease on fetal brain development and injury. Current opinion in pediatrics. 2011;23(5):502-11.
Figures