1070
Multinuclear in vivo MR spectroscopy at 7T reveals differences in liver and muscle metabolism in NAFLD patients and healthy controls1High-field MR Centre, Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Vienna, Austria, 2Division of Endocrinology and Metabolism, Department of Internal Medicine III, Medical University of Vienna, Vienna, Austria, 3Division of Gastroenterology and Hepatology, Department of Internal Medicine III, Medical University of Vienna, Vienna, Austria, 4Christian Doppler Laboratory for Clinical Molecular MR Imaging, Vienna, Austria
Synopsis
Recent developments at 7T allowed for qualitative assessment of the hepatic lipids, faster measurement of glycogen and ATP turnover. Improved spectral resolution also enabled better separation and quantitation of hepatic metabolites. The aim of the study was to employ the advanced methods of multinuclear in vivo MR spectroscopy at 7T in the liver and muscles in non-alcoholic fatty liver disease (NAFLD) patients and healthy controls. Static and dynamic multinuclear MRS data, supported by mixed meal test and euglycemic-hyperinsulinemic clamp study, showed metabolic changes and disturbed interorgan crosstalk in NAFLD patients.
Purpose
Non-alcoholic fatty liver disease (NAFLD) is the hepatic manifestation of the metabolic syndrome. The prevalence of NAFLD is rising worldwide1 and methods for accurate assessment are required. NAFLD is characterized by steatosis, which can be assessed by MRI or proton (1H) MR spectroscopy (MRS). Recent developments at 7T allowed for qualitative assessment of the lipids by calculating unsaturation index of fatty acids (UI)2. NAFLD patients have impaired insulin sensitivity3 which can influence tissue glycogen metabolism. Alterations of tissue glycogen and lipid metabolism in the course of mixed meal test (MMT) were already monitored in patients with type 2 diabetes4 and young insulin resistant population5. On the cellular level, decreased mitochondrial ATP is suspected link to insulin sensitivity in the liver and muscle6. Measurement of hepatic ATP turnover was already applied at 7T to differentiate between healthy, NAFLD and non-alcoholic steatohepatitis7. Moreover, the improved spectral resolution at 7T also enables separation and quantitation of further 31P hepatic metabolites8. The aim of the study was to employ the advanced methods of multinuclear MRS at 7T in the liver and muscles, supported by MMT and euglycemic-hyperinsulinemic clamp study, to obtain profound insight into the NAFLD.Methods
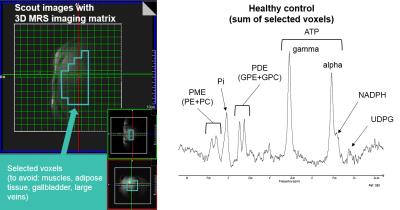
Five non-diabetic NAFLD patients (Age 49.6±2.3years, BMI 29.1±2.2kg.m-2, m/f=3/2, HbA1C<6.5%) and nine healthy controls (Age 27.5±2.1years, BMI 21.50±0.50kg.m-2, m/f=5/4), both groups with alcohol consumption<30g/day, were included in the study. For the assessment of insulin sensitivity two steps euglycemic-hyperinsulinemic clamp in combination with stable glucose isotope tracers [6,6-2H2] was performed. MMT (accompanied by 1H/13C MRS) was performed with test liquid meals containing well balanced and individually adjusted required daily energy intake. The meal was served twice: after the first MRS measurement and 4 hours later, 3 hours before the second MRS measurement. MRS measurements were performed on 7T scanner (Siemens, Germany) with 1H/13C (Stark Contrast, Germany) and 1H/31P (Rapid Biomedical, Germany) surface coils. The MMT and the clamp study were performed at specialized clinical metabolic ward. Blood sugar, insulin, C-peptide and triglycerides were collected from the blood during the MMT. Liver and muscle 1H/13C-MRS was performed twice (morning and evening). 1H-MRS: The HCL and UI was measured and calculated with STEAM sequence (TR/TE=5000/6ms, NA=8)2,9. The intramyocellular lipids (IMCL) were measured in soleus muscle with STEAM sequence (TR/TE=5000/20ms, NA=32, w/o water suppression). 13C-MRS: The hepatic and skeletal muscle glycogen content was measured with pulse-acquire sequence (TR/TE=1000/0.35ms, NA=3x256). The signal was quantified by phantom replacement, calibrated to in vivo load and corrected on sensitive tissue volume. The hepatic 31P spectra were measured with 3D-CSI sequence (matrix size: 16x16x16, TR/TE=1800/0.9ms, NA=1) and the ATP turnover with saturation transfer 1D-ISIS sequence (TR/TE=5000/0.4ms, NA=24)6 in fasting conditions at the day of clamp study and at least 3 days appart from the MMT and 1H/13C MRS measurement. The data were processed with AMARES10. For group difference analysis the t-Test was used.Results
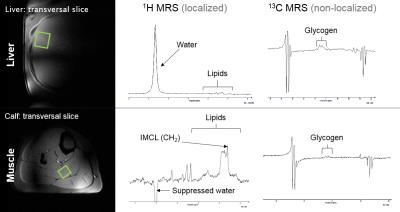
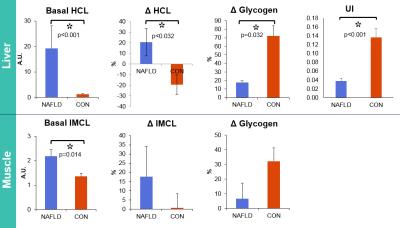
The M-values derived from the clamp study showed 87% decrease in hepatic and 75% decrease in whole body insulin sensitivity in NAFLD patients. The data acquired during MMT demonstrated postprandial hyperlipidemia in NAFLD patients. Typical MRS setting with examples of 1H and 13C spectra is illustrated in Fig.1. Morning 1H-MRS showed increased lipid content in the liver and muscle in NAFLD patients (Basal HCL and IMCL, Fig.2). Postprandial changes revealed increase in lipids accumulation in NAFLD patients (ΔHCL, Fig.2), which was in inverse relationship with glycogen changes (ΔGlycogen, Fig.2) in both tissues. 3D-CSI setting with example of 31P spectrum is illustrated in Fig.3. The 31P-MRS measurements revealed increase of glycerophosphocholine (p=0.02) and decrease of phosphomonoesters (PME, p=0.03) and nicotinamide-adenine-dinucleotide-phosphate (p<0.002) in case of NAFLD patients. The data from ATP turnover (k-values) were not significantly different and resulted in NAFLD patients: 0.22±0.05s-1 and in controls: 0.17±0.02s-1.
Discussion
1H and 13C-MRS data from NAFLD patients displayed impaired glycogen synthesis in the liver and skeletal muscle together with increased ectopic lipid storage in liver and skeletal muscle in the basal and as well in postprandial states. We could show elevated HCL and IMCL and decreased UI in NAFLD patients. Elevated GPC was already found in NAFLD11. The amplitudes of ATP turnover were in agreement with previous study7.Conclusion
Dynamic multinuclear MRS data, supported by metabolic challenges, showed disturbed interorgan crosstalk in NAFLD patients. Employed multinuclear MRS methods at 7T allowed for measurements with high precision, making the multinuclear MRS a tool for outstanding metabolic research.Acknowledgements
The authors wish to thank Astrid Hofer for help with clamp measurements, Tanja Lamp and Christian Rechling MD for recruiting patients and healthy controls, and Vienna Science and Technology Fund (WWTF, LS12-008) for financial support.References
1. Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013 Nov;10(11):686-90.
2. Gajdošík M, Chadzynski GL, Hangel G, et al. Ultrashort-TE stimulated echo acquisition mode (STEAM) improves the quantification of lipids and fatty acid chain unsaturation in the human liver at 7 T. NMR Biomed. 2015 Oct;28(10):1283-93.
3. Utzschneider KM1, Kahn SE. Review: The role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006 Dec;91(12):4753-61.
4. Krssak M, Brehm A, Bernroider E, et al. Alterations in postprandial hepatic glycogen metabolism in type 2 diabetes. Diabetes. 2004 Dec;53(12):3048-56.
5. Petersen KF, Dufour S, Savage DB, et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci U S A. 2007 Jul 31;104(31):12587-94.
6. Szendroedi J, Kaul K, Kloock L, et al. Lower fasting muscle mitochondrial activity relates to hepatic steatosis in humans. Diabetes Care. 2014 Feb;37(2):468-74.
7. Valkovic L, Gajdošík M, Traussnigg S, et al. Application of localized ³¹P MRS saturation transfer at 7 T for measurement of ATP metabolism in the liver: reproducibility and initial clinical application in patients with non-alcoholic fatty liver disease. Eur Radiol. 2014 Jul;24(7):1602-9.
8. Chmelik M, Považan M, Krššák M, et al. In vivo (31)P magnetic resonance spectroscopy of the human liver at 7 T: an initial experience. NMR Biomed. 2014 Apr;27(4):478-85.
9. Gajdošík M, Chmelík M, Just-Kukurová, et al. In vivo relaxation behavior of liver compounds at 7 Tesla, measured by single-voxel proton MR spectroscopy. J Magn Reson Imaging. 2014 Dec;40(6):1365-74.
10. Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson. 1997; 129(1):35-43.
11. Abrigo JM, Shen J, Wong VW, et al. Non-alcoholic fatty liver disease: spectral patterns observed from an in vivo phosphorus magnetic resonance spectroscopy study. J Hepatol. 2014 Apr;60(4):809-15.
Figures