1066
Long-term Effects of Recurrent Neonatal Hyperglycemia on the Hippocampal Neurochemical Profile of Rats.Raghavendra Rao1 and Ivan Tkac2
1Department of Pediatrics, University of Minnesota, Minneapolis, MN, United States, 2Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States
Synopsis
Hyperglycemia is common in extremely low-gestational-age neonates (ELGAN) and increases the risk of serious health problems in the neonatal period. However, the long-term effects are not well understood. The purpose of this study was to assess long-term effects of the recurrent neonatal hyperglycemia on the hippocampal neurochemical profile. Metabolite changes were quantified by in vivo 1H MRS at 9.4T using a rat model of neonatal hyperglycemia. The results of this study indicate that the recurrent hyperglycemia during neonatal period may alter energy metabolism and glutamatergic neurotransmission, which can contribute to delayed hippocampal development and cognitive deficits in ELGANs.
PURPOSE
Hyperglycemia
(blood glucose >150 mg/dL) is common in extremely low-gestational-age
neonates (ELGAN, gestational age 24 - 28 week at birth) with an incidence of 40
- 80% 1,2. Relative
hypoinsulinism combined with the need for dextrose infusion for nutrition are
responsible for hyperglycemia in these infants. Hyperglycemia increases the
risk of death, intraventricular hemorrhage and sepsis in the neonatal period 3. The long-term effects are not well
understood. Limited data demonstrate that recurrent neonatal hyperglycemia is
associated with deceleration of somatic and brain growth that lasts at least
until the age of two years in ELGANs 2,4
and with neurodevelopmental deficits at two years of age 5. The adverse effects were directly
proportional to the severity of hyperglycemia in the neonatal period 2,4.
In other conditions (for example, diabetes mellitus), hyperglycemia
causes structural and functional changes in the hippocampus 6,7. Long-term structural and functional
hippocampus-specific deficits are common in ELGANs 8. It is not known whether hyperglycemia has a role in these
deficits. The purpose of the study was to determine whether recurrent
hyperglycemia in the neonatal period leads to long-term alterations in the
neurochemical profile of the hippocampus using a rat model of neonatal
hyperglycemia.METHODS
Sprague-Dawley rat pups were subjected to hyperglycemia of 2-hour duration, twice daily from postnatal day 3 (P3, stage of brain development = human ELGAN) to P12 (stage of brain development = human full term neonate) using subcutaneous injection of 30% dextrose (moderate hyperglycemia - MODERATE-HG) group or 50% dextrose (severe hyperglycemia - SEVERE-HG) group. Littermates in the control group (CONTROL) were injected with normal saline. The neurochemical profiles of the hippocampus were determined in the CONTROL (N = 6), MODERATE-HG (N = 6) and SEVERE-HG (N = 7) group on P30 (stage of brain development = 2-year-old human child) using 1H MRS at 9.4T. Data were acquired using FASTMAP shimming 9 and ultra-short TE STEAM (TE = 2 ms) localization sequence combined with VAPOR water suppression 10. Metabolites were quantified using LCModel with the spectrum of fast relaxing macromolecules included in the basis set. Spontaneously breathing animals were anesthetized with 1.0 – 1.5% isoflurane. Brain tissue was harvested and processed for histochemical assessment of synaptic structure (b-tubulin histochemistry) and astrocytosis (S100b histochemistry). The intergroup differences in metabolite concentrations and histochemistry were determined using ANOVA and t-tests.RESULTS
The blood glucose concentration (mg/dL) was 139 ± 3, 226 ± 12 and 380 ± 27 in the CONTROL group, MODERATE-HG group and SEVERE-HG group, respectively (p < 0.01). The spectral quality consistently accomplished in this study (Fig. 1) enabled reliable quantification of seventeen brain metabolites (Fig. 2). Small but significant changes (p < 0.05) were observed for lactate concentration as well as for glutamate/glutamine and phosphocreatine/creatine ratios in hyperglycemia groups relative to controls. The Lac levels and Glu/Gln ratios progressively decreased with the severity of the neonatal hyperglycemia. The PCr/Cr ratio increased in hyperglycemia groups relative to controls. Histochemistry demonstrated decreased dendritic arborization density (lower β-tubulin density) and increased astrocytosis (higher number of S100b-positive cells) in the hippocampus of the MODERATE-HG and SEVERE-HG groups.DISCUSSION
Recurrent neonatal hyperglycemia altered the neurochemical profile and structure of the developing rat hippocampus. The presence of changes at P30, despite cessation of hyperglycemia on P12, indicates that neonatal hyperglycemia may cause long-term effects in the developing brain. Observed changes in metabolites directly involved in brain energy metabolism (PCr/Cr, Lac) indicate decreased hippocampal activity 11. Moreover, decreased Glu/Gln ratio implies altered glutamate-glutamine cycling between neurons and glia 11. Alterations in energy metabolism and glutamate-glutamine cycling are in a good agreement with the decreased synaptic density observed in the hyperglycemia groups. Astrocytosis may also contribute to the lower Glu/Gln ratio in the hyperglycemia group, since Glu and Gln are primarily localized in neuronal and astrocytic compartments, respectively. A similar pattern of neurochemical changes has been linked to cognitive impairments in adults with Alzheimer disease 12,13.CONCLUSIONS
The results of this study indicate that the recurrent hyperglycemia during neonatal period may alter energy metabolism and glutamatergic neurotransmission, which can contribute to delayed hippocampal development and cognitive deficits in ELGANs.Acknowledgements
Supported by: NIH grants P41 EB015894, P30 NS076408 and WM KECK FoundationReferences
1. Beardsall K, Vanhaesebrouck S, Ogilvy-Stuart AL, Vanhole C, Palmer CR, Ong K, vanWeissenbruch M, Midgley P, Thompson M, Thio M, Cornette L, Ossuetta I, Iglesias I, Theyskens C, de Jong M, Gill B, Ahluwalia JS, de Zegher F, Dunger DB. Prevalence and determinants of hyperglycemia in very low birth weight infants: cohort analyses of the NIRTURE study. J Pediatr 2010; 157: 715-719 e711-713. 2. Ramel SE, Long JD, Gray H, Durrwachter-Erno K, Demerath EW, Rao R. Neonatal hyperglycemia and diminished long-term growth in very low birth weight preterm infants. J Perinatol 2013; 33: 882-886. 3. Kao LS, Morris BH, Lally KP, Stewart CD, Huseby V, Kennedy KA. Hyperglycemia and morbidity and mortality in extremely low birth weight infants. J Perinatol 2006; 26: 730-736. 4. Scheurer JM, Gray HL, Demerath EW, Rao R, Ramel SE. Diminished growth and lower adiposity in hyperglycemic very low birth weight neonates at 4 months corrected age. J Perinatol 2016; 36: 145-150. 5. van der Lugt NM, Smits-Wintjens VE, van Zwieten PH, Walther FJ. Short and long term outcome of neonatal hyperglycemia in very preterm infants: a retrospective follow-up study. BMC Pediatr 2010; 10: 52. 6. Perantie DC, Lim A, Wu J, Weaver P, Warren SL, Sadler M, White NH, Hershey T. Effects of prior hypoglycemia and hyperglycemia on cognition in children with type 1 diabetes mellitus. Pediatr Diabetes 2008; 9: 87-95. 7. Hershey T, Perantie DC, Wu J, Weaver PM, Black KJ, White NH. Hippocampal volumes in youth with type 1 diabetes. Diabetes 2010; 59: 236-241. 8. Gimenez M, Junque C, Narberhaus A, Caldu X, Salgado-Pineda P, Bargallo N, Segarra D, Botet F. Hippocampal gray matter reduction associates with memory deficits in adolescents with history of prematurity. Neuroimage 2004; 23: 869-877. 9. Gruetter R, Tkac I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn Reson Med 2000; 43: 319-323. 10. Tkac I, Starcuk Z, Choi IY, Gruetter R. In vivo H-1 NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med 1999; 41: 649-656. 11. Raman L, Tkac I, Ennis K, Georgieff MK, Gruetter R, Rao R. In vivo effect of chronic hypoxia on the neurochemical profile of the developing rat hippocampus. Brain Res Dev Brain Res 2005; 156: 202-209. 12. Trushina E, Dutta T, Persson XM, Mielke MM, Petersen RC. Identification of altered metabolic pathways in plasma and CSF in mild cognitive impairment and Alzheimer's disease using metabolomics. PLoS One 2013; 8: e63644. 13. Rupsingh R, Borrie M, Smith M, Wells JL, Bartha R. Reduced hippocampal glutamate in Alzheimer disease. Neurobiol Aging 2011; 32: 802-810.Figures
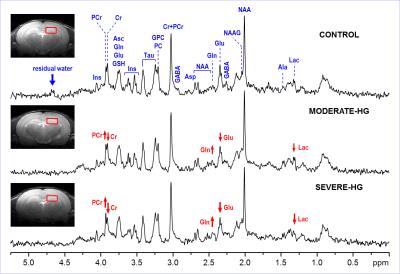
Representative
in vivo 1H MR spectra
acquired on postnatal day (P) 30 from the hippocampus of rats exposed to
recurrent neonatal hyperglycemia (P3 – P12). STEAM (B0 = 9.4 T, TE =
2 ms, TR = 5 s), coronal FSE images show the VOI selection. Post-processing
water signal removal or baseline correction has not been applied.
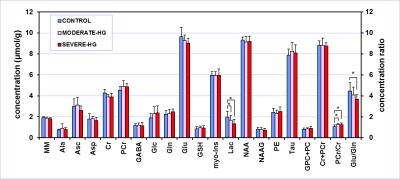
Hippocampal
neurochemical profiles of P30 rats exposed to recurrent neonatal hyperglycemia
(2-hour duration, 2 times per day, P3 – P12) and their controls. Moderate
hyperglycemia was induced by subcutaneous
injection of 30% dextrose; severe hyperglycemia was induced by 50% dextrose. T-test,
* p < 0.05, error bars indicate SDs.