1065
The effect of high-intensity interval training on brain lactate levels during subsequent hypoglycemia in type 1 diabetes1Radiology and Nuclear Medicine, Radboud university medical center, Nijmegen, Netherlands, 2Internal Medicine, Radboud university medical center, Nijmegen, Netherlands, 3Pediatrics, Radboud university medical center, Nijmegen, Netherlands
Synopsis
Adaptations in brain lactate handling in response to hypoglycemia may play a role in the pathophysiology of impaired awareness of hypoglycemia (IAH). Therefore, we determined the effect of high-intensity interval training (HIIT)-induced hyperlactatemia on brain lactate during subsequent hypoglycemia in type 1 diabetic (T1DM) patients with IAH, in T1DM patients with normal awareness of hypoglycemia (NAH) and in healthy controls. Brain lactate concentration was determined using a J-difference editing semi-LASER 1H-MRS sequence. After HIIT, brain lactate concentration increased most in T1DM IAH, consistent with an enhanced lactate transport capacity, and also dropped most during subsequent hypoglycemia, suggestive for increased lactate oxidation.
Introduction
Hypoglycemia, a frequent side-effect of insulin therapy in type 1 diabetes (T1DM), normally elicits well-known symptoms that allow patients to take corrective actions. However, in about 25-30% of the T1DM patients, hypoglycemic symptoms are suppressed. This condition is referred to as impaired awareness of hypoglycemia (IAH) and is a high risk for severe, potential hazardous, hypoglycemia. Previous studies suggest involvement of alterations in brain lactate handling in response to hypoglycemia in the pathophysiology of IAH.1-3
We have shown that brain lactate levels drop in response to hypoglycemia in T1DM IAH1, consistent with increased lactate oxidation when glucose supply is low. High-intensity interval training (HIIT) increases plasma lactate levels. When such excess lactate is transported into the brain, it may affect awareness of hypoglycemia.4,5 Therefore, we aimed to assess the effect of HIIT on cerebral lactate during hypoglycemia in patients with IAH and compared results to those obtained in patients with normal awareness of hypoglycemia (NAH) and in healthy controls (HC).
Methods
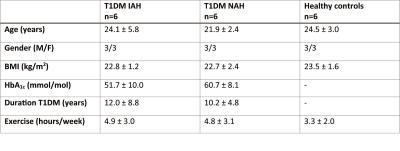
Experimental protocol: Six T1DM IAH subjects, six T1DM NAH subjects and six HC (table 1) underwent a hypoglycemic hyperinsulinemic clamp (plasma glucose of 2.8 mmol/l for 45-60 minutes) directly after performing HIIT. HIIT consisted of three 30-second all-out sprints on a cycle ergometer interspersed with 4 minutes active recovery. Before HIIT (baseline) and during hypoglycemia, brain lactate levels were determined continuously with 1H-MRS at 3T (Tim MAGNETOM Trio, Siemens) using a J-difference editing semi-LASER sequence6,7 (TE 144 ms, TR 3000 ms, 32 averages). J-difference editing was performed with frequency selective inversion pulses (MEGA, bandwidth of 75 Hz) centered on the lactate quartet at 4.1 ppm and subsequently at -3 ppm. Additionally, spectra without water suppression (TE 30 ms, TR 5000 ms, 8 averages) were acquired. Data were acquired from a 22.5 cm3 voxel placed in the periventricular brain region. Arterial plasma glucose and lactate levels were determined every 5 minutes. Subjects completed a semi-quantitative symptom questionnaire at baseline and at the end of the hypoglycemic phase.
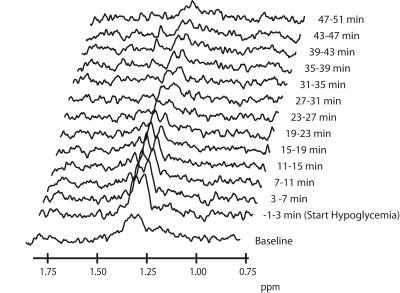
Post-processing: After zero-filling from 1024 to 2048 points and Fourier transformation, spectra were phase and frequency aligned with the first spectrum recorded. The J-difference edited spectra were subtracted pairwise and subsequently apodized in the time-domain with a 5-Hz Lorentzian. For a better signal-to-noise ratio per spectrum, a 3-point moving average filter was applied. In the final difference spectra (Figure 1), the lactate doublet was fitted with the AMARES algorithm in jMRUI8. Cerebral lactate was quantified using the unsuppressed water signal as a reference, taking voxel composition, differences in T2 relaxation and the contribution of plasma lactate into account. Time courses of cerebral lactate were fitted with an exponential decay function using a least-squares fit algorithm, according to the following equation:
$$Lac(t) = (Lac_{0}-Lac_{Plateau}) *e^{-k*t}+Lac_{Plateau}$$
Were Lac0 is the cerebral lactate level after HIIT, k the rate constant and LacPlateau the steady-state difference between baseline and the end of hypoglycemia.
Statistics: Significant differences on calculated or fitted values between conditions and groups were determined with a T-test or ANOVA (with Bonferonni post-hoc testing), respectively.
Results
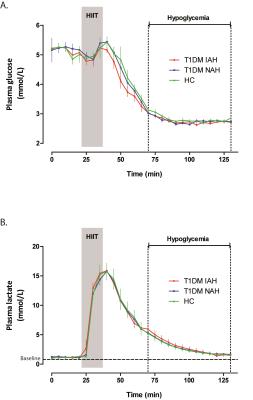
Figure 2 shows the time courses of plasma glucose and plasma lactate for the three groups of subjects. As expected, HIIT markedly increased plasma lactate levels. After reaching peak levels, plasma lactate levels fell gradually but remained above baseline levels during subsequent hypoglycemia. Hypoglycemic symptom scores increased significantly in response to hypoglycemia in HC (+10.8±5.0) and in T1DM NAH (+14.8±8.8), but not in T1DM IAH (+0.7±3.1, p<0.01 versus the other groups).
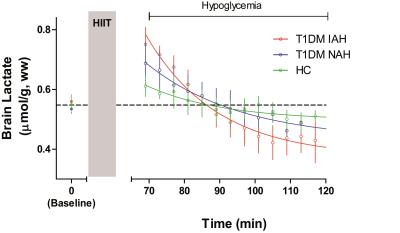
Brain lactate levels were similar at baseline across the three groups (0.56±0.05 µmol/g,ww, 0.53±0.03 µmol/g,ww and 0.55±0.05 µmol/g,ww in T1DM IAH, T1DM NAH and HC respectively). Brain lactate increased after HIIT to 0.76±0.04 µmol/g, ww in T1DM IAH and to a lesser extent in T1DM NAH (0.68±0.04 µmol/g, ww) and HC (0.61±0.04 µmol/g,ww; p<0.05 between all groups). During hypoglycemia, brain lactate levels returned to and remained at baseline levels in T1DM NAH and HC, whereas a -0.19±0.08 µmol/g,ww decrease below baseline (p<0.01; Figure 3) was observed in T1DM IAH at the end of hypoglycemia (LacPlateau).
Discussion and Conclusion
In patients with T1DM and impaired awareness of hypoglycemia, brain lactate uptake after HIIT is higher and brain lactate levels drop to a lower steady-state level during subsequent hypoglycemia, as compared to patients with T1DM and normal awareness of hypoglycemia and healthy controls. These results are consistent with the previously reported upregulation of lactate transporters9,10 and an increased cerebral lactate oxidation during hypoglycemia. Both alterations may contribute to (the persistence of) impaired awareness of hypoglycemia.Acknowledgements
No acknowledgement found.References
1. Wiegers et al. Diabetes 2016; 2. Herzog et al. J Clin Invest 2013 3. de Feyter et al. Diabetes 2013 4. Maran et al. Diabetologia 2000 5. Veneman et al. Diabetes 1994 6. Star-Lack et al. J Magn Reson, 1998 7. Scheenen et al. Magn Reson Med, 2008 8. Vanhamme et al. J Magn Reson, 1997 9. Mason et al. Diabetes 2006 10. Gulanski et al J Clin Endocrinol Metab, 2013Figures