1064
Transcranial direct current-induced modulation of GABA levels and resting-state functional connectivity in older subjects1Physikalisch-Technische Bundesanstalt (PTB), Berlin, Germany, 2Department of Neurology, NeuroCure Clinical Research Center, Charité, Berlin, Germany, 3Center for Stroke Research, Charité, Berlin, Germany
Synopsis
Transcranial direct current stimulation (tDCS) modulates human behavior, neuronal patterns and metabolite concentrations. To unravel tDCS-induced alterations on the neuronal level we investigated tDCS-induced effects in older adults (50-79 years) using MRS to quantify GABA levels and resting-state fMRI to assess sensorimotor network strength and inter-hemispheric connectivity. Anodal, cathodal and sham tDCS were applied over the left sensorimotor region in a randomized, cross-over design. Compared to sham, anodal tDCS induced significantly reduced GABA levels, representing local plasticity, as well as lower large-scale network coupling and inter-hemispheric connectivity.
Introduction
Transcranial direct current stimulation (tDCS) modulates human behavior, neuronal patterns and metabolite concentrations.1-3 Anodal tDCS has been found to induce reduced GABA levels within primary sensorimotor cortices4-6 and the strength of this effect correlated positively with learning and memory in the motor domain. In young adults, widespread resting-state functional connectivity (FC) changes were reported as a result of tDCS, with observations of augmented coupling within networks of interest.4 However, because of substantial alterations in the older human brain with regard to structure, connectivity, neurotransmitter levels, and the ability to induce long-term potentiation, stimulation effects often differ substantially from those in young healthy brains. Here, a systematic investigation of tDCS-induced effects in older adults was aimed at, using MRS to measure GABA levels and resting-state fMRI to assess functional connectivity.Methods
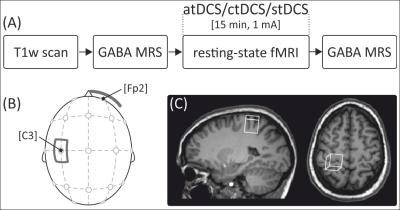
Forty-eight healthy older adults (50-79 years, 24 female) participated in three scanning sessions with either anodal, cathodal or sham tDCS. Experiment design and stimulation setup are depicted in Figure 1. Measurements were performed on a 3T scanner (Verio, Siemens, Germany) using a 32-channel head coil. Following MPRAGE imaging and FAST(EST)MAP shimming, MEGA-PRESS spectra were acquired in a 22 × 22 × 22 mm³ voxel placed in left SM1 (Fig. 1C) before and after stimulation. GABA concentration was quantified relative to total creatine whose levels were not affected by the stimulation. FC was assessed using resting-state fMRI during stimulation. Seed-based and independent-component-analysis based approaches were used to assess tDCS-induced changes of inter-hemispheric FC and coupling strength within the sensorimotor network (SMN).Results
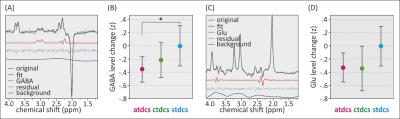
A sample magnetic resonance spectrum for GABA is depicted in Figure 2. Relative GABA changes between conditions were determined by mixed-model analyses including the factor condition and the covariate age as well as their interaction (Fig. 2B). The main effect of condition was significant (p < 0.05). Post-hoc comparisons of model-based means showed that the GABA level was significantly reduced in (anodal) atDCS compared to sham. This effect was polarity specific as there was no significant difference between (cathodal) ctDCS and sham. The effect of age was not significant, but there was a significant interaction between condition and age. To explore the meaning of this interaction, we divided the age group using a median split (<63y: young-old (YO), >63y: old-old (OO)). This distinction revealed that the atDCS effect was larger in the OO group (F(2,87)=5.54, p=.005). Repeating this analysis with glutamate levels instead of GABA showed no statistically significant effect of condition or age (Fig. 2D). Post-hoc comparisons demonstrated a significantly lower inter-hemispheric FC and reduced SMN strength in atDCS compared to sham. At baseline, GABA levels are inversely correlated to overall SMN strength but no significant relationship is observed between tDCS-induced GABA changes and SMN strength. These findings are consistent with previous studies4,7 on younger subjects.Discussion
This is the first study in older adults, spanning a wide age range (50 to 79 years), showing tDCS-induced plasticity in local inhibitory tone of the sensorimotor cortex in combination with resting-state FC. Local inhibitory tone was assessed using MRS to measure GABA levels in left SM1, a major node of the SMN and the target of the stimulation. First, we observed a significant reduction of GABA levels after atDCS compared to sham, reflecting tDCS-induced neuroplastic alterations in brain chemistry. Second, resting-state functional coupling was decreased during atDCS compared to sham, most likely indicating augmented efficiency in brain network functioning. This decrease was evident in both inter-hemispheric FC and SMN strength. Third, while an association between higher baseline levels of functional coupling and lower GABA levels was observed, the magnitudes of tDCS-induced effects on these parameters were not correlated. Lastly, exploring an association of atDCS-induced GABA changes with baseline SMN strength revealed opposite directions in the younger compared to the older subgroup, suggesting age-related differences in network integrity. Consistent with previous studies in young adults, we observe that anodal tDCS over the left SM1 significantly reduces GABA levels within the target region compared to sham. The reduction is neurochemically specific, as no tDCS-effect was observed on glutamate, and polarity specific, as it was not observed after cathodal tDCS.Conclusion
The observation of reduced GABA after atDCS suggests (1) the involvement of GABAergic neurotransmission in the neuronal effects of atDCS (targeting the SMN) in older adults and (2) preserved plasticity of the sensorimotor system in older adults, similar to what has been reported for younger adults.Acknowledgements
No acknowledgement found.References
1. Fertonani A, Miniussi C. 2016. Transcranial Electrical Stimulation: What We Know and Do Not Know About Mechanisms. Neuroscientist. doi: 10.1177/1073858416631966.
2. Nitsche MA, Polania R, Kuo MF. 2015. Transcranial Direct Current Stimulation: Modulation of Brain Pathways and Potential Clinical Applications. In Brain Stimulation, 233-254. John Wiley & Sons, Inc.
3. Perceval, G., A. Floel, and M. Meinzer. 2016. Can transcranial direct current stimulation counteract age-associated functional impairment? Neurosci Biobehav Rev 65:157-72.
4. Bachtiar V, Near J, Johansen-Berg H, Stagg CJ. 2015. Modulation of GABA and resting state functional connectivity by transcranial direct current stimulation. Elife 4:e08789.
5. Stagg CJ, Best JG, Stephenson MC, O'Shea J, Wylezinska M, Kincses ZT, Morris PG, Matthews PM, and Johansen-Berg H. 2009. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J Neurosci 29 (16):5202-6.
6. Kim S, Stephenson MC, Morris PG, and Jackson SR. 2014. tDCS-induced alterations in GABA concentration within primary motor cortex predict motor learning and motor memory: a 7 T magnetic resonance spectroscopy study." Neuroimage 99:237-43.
7. Stagg CJ. 2014. Magnetic Resonance Spectroscopy as a tool to study the role of GABA in motor-cortical plasticity." Neuroimage 86:19-27.
Figures