1044
Dynamic imaging of eye and optic nerve with golden angle radial MRI.1Department of Radiology, Vanderbilt University Medical Center, Nashville, TN, United States, 2Vanderbilt University Institute of Imaging Science, 3Department of Biomedical Engineering, Vanderbilt University, Nashville, TN, United States
Synopsis
In this abstract we present dynamic imaging of the eyes and the optic nerves in humans using golden angle radial MRI. Continuous 15 s radial scans with azimuthal profile steps of 111.246 degrees are acquired under various eye motion states. Qualitative analyses of the images reveal features of basic eye and nerve mechanics. Image-based characterization of eye mechanics can improve understanding eye physiology and disease.
INTRODUCTION
The eyes and optic nerves make up the primary sensory segments of the human anterior visual pathway, yet have been exceptionally challenging to image directly. In-vivo MRI of the eyes and optic nerves has largely been limited to the stationary eye with ocular fixation to stabilize the nerve1,2, since motion is a major confound rather than the point of interest. However, imaging dynamic eye and nerve motion can be potentially valuable in understanding basic eye mechanics as well as pathologies of the visual system. Aberrations in these dynamics can serve as early indicators of disease3,4 and combined with traditional radiological markers give a complete picture of visual system sensory function. In this abstract, we present the use of Golden angle (GA) radial MRI5 for imaging the moving eye and optic nerve at 3 Tesla. GA radial MRI has recently been employed for several dynamic imaging applications, including imaging of speech6, joints7 and the abdomen8 but has never been applied to dynamically survey the orbit in humans.METHODS
Acquisition: All experiments were performed on a Philips Achieva 3 Tesla human imaging system (Philips Healthcare) with a 2-channel body transmit / 8 channel head receive coil. Two healthy subjects were scanned after signed informed consent. One 8 mm axial, coronal or sagittal slice was planned based on T2 weighted scout images, such that the complete extent of the nerve was captured in the axial and sagittal slices along with the eyes. Data were acquired with a GA radial imaging sequence in which the radial spokes were stepped by 111.246 degrees without readout alternation. Data were acquired continuously for ~15 seconds with a TR/TE of 5.7/1.4 ms, flip angle of 20 degrees, in-plane FOV of 200 mm and a readout resolution of 1 mm. To probe eye and optic nerve dynamics, the subjects were instructed to perform various eye movements during the scan. These included 1. Resting 2. Both eyes blinking 3. Sweeping eyes from left to right and back. 4. Sweeping eyes up and down 5. Fixating on a cross on a screen.
Reconstruction: Complex axial image volumes were reconstructed offline in Python (Anaconda version 3.4.2, TX) using 128 profiles per reconstructed slice (0.73 s temporal span) and a window step of 10 radial spokes (0.057 ms per frame). GA image reconstruction involved correction for k-space shifts and sampling density prior to gridding, 2D FFT and roll-off compensation9. Channel combination was performed in the image domain using the method of Walsh et al.10 to yield final dynamic images.
RESULTS
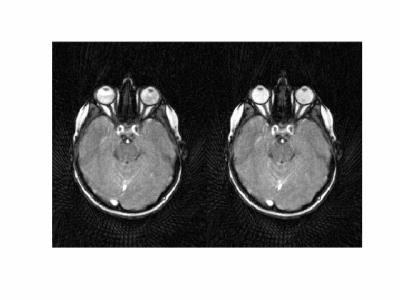
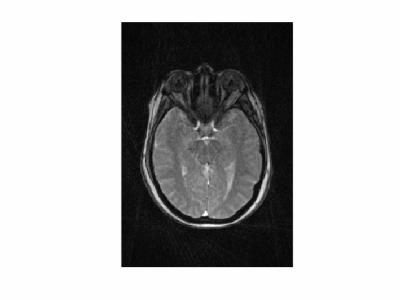
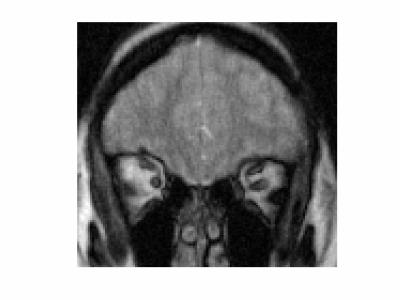
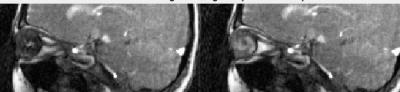
Dynamic image data are presented here as animated .gif files. Note that the images have been compressed to create animated .gif files under 2 Mb with some loss of quality. Figure 1 shows images from volunteer 1, at rest (left) and with eyes fixated (right). The entire orbital anatomy is seen with good contrast between the eye globes, optic nerve, surrounding fat and the medial and lateral rectus muscles. Qualitative observation of the two states reveals that there is involuntary drifting motion of the nerve in the resting state, which is minimized by fixation. Although somewhat expected, this data can help improve the design of imaging experiments in the eye where motion is a challenge. Assessment of eye fixation and drift may also be useful in evaluating disease states. Figures 2 and 3 show data from volunteer 2 sweeping eyes left-right in the axial (Fig 2) and coronal (Fig 3) orientations. Abduction/adduction of the medial/lateral rectus muscles impart large movements of the eyes along with the optic nerves. Hyperintense signal inside the eye globes are also seen to change with motion in Figure 2, which we hypothesize originate from swirling flow of vitreous humor. This hyperintensity is seen to originate circumferentially with each eye movement. Figure 4 shows blinking (left) and up-down sweeping (right) of the eye in the sagittal plane. Muscle movements are again observed, as are swirling motions of the vitreous fluid. Figure 5 shows blinking of both eyes in the axial plane.DISCUSSION
Imaging of eye and optic nerve dynamics can be useful in understanding basic eye function as well as a range of pathologies. GA MRI allows the flexible retrospective reconstruction of image frames to visualize the motion at variable frame rates and image qualities. Images may be reconstructed at a higher frame rate to analyze fast sweeping motions, such as that seen in Figure 3. GA imaging is also amenable to compressed sensing based reconstructions, which will allow further acceleration without large penalties on image quality.Acknowledgements
This work was supported in part by funding from NIH R01 EY023240, W81XWH-13-1-0073 (Smith), NIH/NINDS R21NS087465, and National MS SocietyReferences
1. Hickman SJ, Miszkiel KA, Plant GT et al, The optic nerve sheath on MRI in acute optic neuritis. Neuroradiology 2005; 47:51-55
2. Jeong Ha-Kyu, Dewey BE, Hirtle JAT et al. Improved Diffusion Tensor imaging of the optic nerve using Multi-shot two dimensional navigator acquisitions. Magn Reson Med 2015; 74:953-963.
3. Trillenberg P, Lencer R, Heide W. Eye movements and psychiatric disease. Curr Opin Neurol. 2004; 17(1): 43-7.
4. MacAskill MR, Anderson TJ. Eye movements in neurodegenerative diseases. Curr Opin Neurol. 2016;29(1): 61-8.
5. Winkelmann S et al. IEEE TMI 26.68 (2007).
6. Lingala SG, Sutton BP, Miquel ME. et al. Recommendations for real-time speech MRI. J Magn Reson Imaging. 2016;43(1):28-44.
7. Hopfgartner AJ, Tymofiyeva O, Ehses P et al. Dynamic MRI of the TMJ under physical load. Dentomaxillofac Radiol. 2013; 42(9):20120436.
8. Chandarana H, Feng L, Block TK, et al. Free-breathing contrast-enhanced multiphase MRI of the liver using a combination of compressed sensing, parallel imaging, and golden-angle radial sampling. Invest Radiol. 2013 ;48(1):10-6.
9. Sengupta S, Smith DS, Welch EB. Continuously moving table MRI with golden angle radial sampling Magn Reson Med. 2015 Dec;74(6):1690-7.
10. Walsh DO, Gmitro AF, Marcellin MW. Adaptive reconstruction of phased array MR imagery. Magn Reson Med. 2000;43(5):682-90.
Figures