1040
Novel 3D Yarn-Ball k-space Acquisition for Fast High-Resolution T1-Weighted Brain Imaging1Biomedical Engineering, University of Alberta, Edmonton, AB, Canada
Synopsis
Novel 3D Yarn-Ball k-space acquisition is applied for the first time in vivo with application to T1-weighted human brain imaging and a goal of 0.9x0.9x0.9 mm3 voxels in ~2 minutes at 3T. This efficient, large-looping trajectory allows much more of k-space to be sampled following each excitation than the 1 line of 3D-Gradient-Echo (3D-GRE), facilitating longer repetition times (TR), greater acquisition duty cycle, and full sampling. We demonstrate that as a result images created with Yarn-Ball yield better resolution phantom element distinction, and considerably higher SNR (2x on average) in vivo than the three different 3D-GRE methods tested.
Introduction
3D-Gradient-Echo k-space acquisition (3D-GRE) has many uses including T1-weighted brain imaging, however, this basic rectilinear technique requires many excitations to fill the large 3D matrix associated with high-resolution imaging. To limit scan duration, very short readout times (TRO) are implemented in combination with short repetition times (TR) and under-sampling. Each aspect adversely affects the signal (or contrast) to noise ratio (SNR, CNR) in the image. Here we present an alternative to 3D-GRE for T1-weighted human brain imaging – the first in vivo application of our 3D Yarn-Ball technique1. This technique efficiently traverses 3D k-space with mostly large loops allowing rapid acquisition with large gradients and much more than 1 line of k-space to be sampled following each excitation, facilitating both longer TR and greater acquisition duty cycle while negating the need for parallel imaging. Our goal was the acquisition of 0.9x0.9x0.9 mm3 whole head images in ~2 minutes at 3T.Methods
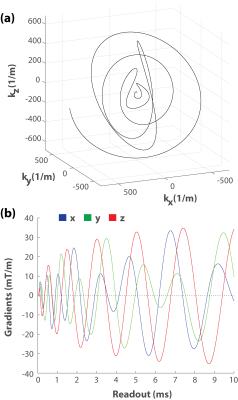
Yarn-Ball was implemented on a Siemens Prisma 3T with 80 mT/m gradients and maximum gradient slew rates of 200 mT/m/ms. 9248 sampling trajectories each 10 ms in duration enabled full sampling support for 0.9x0.9x0.9 mm3 voxels (1/k-space volume) and a 220 cm isotropic FoV in 2:10 minutes (Figure 1). The maximum gradient amplitude was 41 mT/m and the maximum slew rate was 199 mT/m/ms. Note that more rapid gradient change occurs in the small loops nearer the centre of k-space at the start of each trajectory where the gradient amplitudes are smaller. As a result, the peripheral nerve stimulation threshold is not exceeded (i.e. the scanner does not enter first-level controlled operation). The inclusion of (1-1) water excitation to minimize signal from fat, and the constant gradient spoiling of each trajectory resulted in a TR of 13.8 ms. RF spoiling and a flip-angle of 22o was selected for CNR enhancement between gray and white matter at 3T. TE was 75 μs (measured from the second water excitation pulse). A sampling dwell time of 2 μs resulted in the acquisition of ~46 million data points per receiver channel (a 20 element head array coil was used). Gridding was performed on a Titan Black (NVIDIA) graphics card and each coil element image required ~2.5 minutes to create (~45 minutes total time).
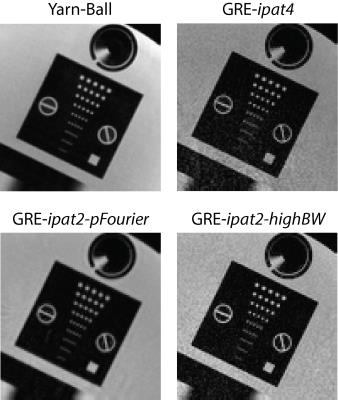
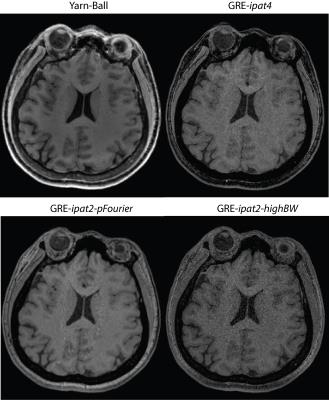
3D-GRE was also considered for the imaging goal described above, and quantized parameter selection resulted in an image matrix of 256x256x192 for a FoV of 224x224x172 (0.88x0.88x0.9 mm3). Images were acquired with a sagittal orientation. Three cases that enabled 2:10 minute scan times were tested. The first used 2x under-sampling in both phase encode directions (4x total), 180 Hz/pix sampling, TE=3.8 ms, TR=8.1 ms, and flip-angle=12o (labeled GRE-ipat4). The second used 2x in-plane under-sampling, 6/8 partial Fourier, 240 Hz/pix sampling, TE=3.1 ms, TR=6.7 ms, and flip-angle=11o (labeled GRE-ipat2-pFourier). The third used 2x in-plane under-sampling, 810 Hz/pix sampling, TE=1.9 ms, TR=4.1 ms, and flip-angle=10o (labeled GRE-ipat2-highBW). Each GRE image used Q-fat fat-suppression and Caipirinha based parallel imaging. Yarn-Ball and 3D-GRE images were acquired from both a Philips resolution phantom and 3 healthy volunteers.
Results
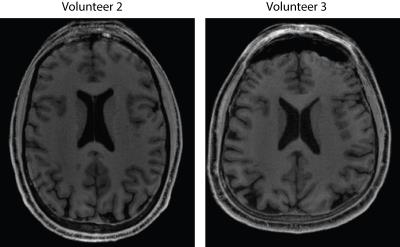
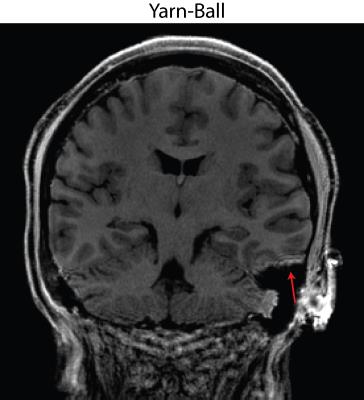
The capability to distinguish resolution elements is clearly superior for Yarn-Ball than any of the 3D-GRE acquisitions (Figure 2). Elevated noise obscures the resolution elements for both GRE-ipat4 and GRE-ipat2-highBW, and the GRE-ipat2-pFourier image appears to have greater blurring as well as some image artifacts. Substantially greater SNR on the Yarn-Ball human brain images is also clearly evident (Figure 3). Measurements in the centrum semiovale yield SNR estimates of [40,36,31], [17,14,12], [31,28,23] and [11,9,9] for Yarn-Ball, GRE-ipat4, GRE-ipat2-pFourier, and GRE-ipat2-highBW respectively (values for all 3 volunteers given). Reproducibility is demonstrated with images from volunteers 2 and 3 (Figure 4). However, Yarn-Ball does also have an Achilles heel, off-resonance, the consequence of which is localized ringing (Figure 5).Discussion and Conclusion
Modern MRI protocols emphasize speed and to that end we have introduced Yarn-Ball, which facilitates the acquisition of 0.9x0.9x0.9 = 0.73 mm3 voxel T1-weighted images in only ~2 minutes at 3T. In general, the quality of the Yarn-Ball images appears to be superior to that afforded by GRE, and future efforts will consider B0 correction to suppress the off-resonance artifact2. Yarn-Ball is also expected to benefit from modern under-sampling advances3, and further scan time reduction is possible. Rigorous comparison with other commonly used sequences is also necessary, as is comparison with other non-Cartesian techniques such as 3D-Cones4. Finally, Yarn-Ball acquisition has potential application for the many other imaging scenarios that currently use 3D-GRE.Acknowledgements
Canadian Institute of Health Research (CIHR)References
1. Stobbe R.W., Beaulieu C., Rapid 3D Spoiled Steady-State Imaging with Yarn-Ball Acquisition, ISMRM, Toronto, 2014.
2. Baron C.A., Nishimura D.G., B0 Mapping Using Rewinding Trajectories (BMART), MRM, 2016, Epub ahead of print
3. Wright K.L., Hamilton J.I., Griswold M.A., Gulani V., Seiberlich N., Non-Cartesian Parallel Imaging Reconstruction, JMRI, 2014, 40:1022-1040
4. Gurney P.T., Hargreaves B.A., Nishimura D.G., Design and analysis of a practical 3D cones trajectory. MRM, 2006, 55:85-91
Figures