1038
Variable Density Single-Shot Fast Spin Echo with Auto-Calibrated Wave Encoding1Electrical Engineering, Stanford University, Stanford, CA, United States, 2Global MR Applications and Workflow, GE Healthcare, Menlo Park, CA, United States, 3Radiology, Stanford University, Stanford, CA, United States, 4Global MR Applications and Workflow, GE Healthcare, Houston, TX, United States
Synopsis
Wave encoding was implemented in a variable-density single-shot fast spin echo (VD-SSFSE) pulse sequence. Auto-calibrated estimation of the wave-encoding point-spread function (PSF) and coil sensitivity maps was used. Images were reconstructed with parallel imaging and compressed sensing reconstruction. Compared to non-wave-encoded Cartesian imaging, wave-encoded VD-SSFSE achieves improved image quality with reduced aliasing artifacts at higher acceleration factors and with full k-space coverage, providing fast acquisitions and clinically relevant echo times.
Purpose
Single-shot fast spin echo (SSFSE) can provide excellent T2 contrast and good motion-robustness for abdominal and pelvic MRI. Variable density (VD) sampling and variable refocusing flip (VRF) angles along the echo train1 have been shown to reduce scan time, enable compressed sensing reconstruction, and allow full k-space coverage with clinically relevant echo times. However, the maximum acceleration factor of variable density sampling is usually limited by the number of available coil elements in the phase-encoding (PE) direction, and therefore, residual aliasing and noise amplification can be observed in highly accelerated VD-SSFSE scans. In this work, we combine wave encoding2 with VD-SSFSE to enable higher acceleration factors. By applying sinusoidal modulation in the PE direction during readout, the proposed wave-encoded VD-SSFSE can reduce noise amplification and residual aliasing artifacts without decreasing the scanning efficiency of conventional VD-SSFSE scans.Methods
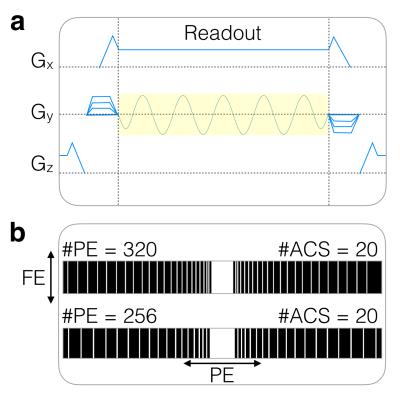
A sinusoidal wave-encoding gradient (5.5 cycles, 12 mT/m amplitude), played out during the readout of each kx encoding line, was added to a VD-SSFSE pulse sequence (Fig. 1a). VD under-sampling patterns with 20 central PE lines of auto-calibration signals (ACS), 50-70 ky views, and an effective acceleration factor of about 5 were used (Fig. 1b). Over-sampling of 1.6-2.0 in the frequency-encoding (FE) direction was used to account for voxel spreading effects due to wave encoding2. The VRF schedule was controlled by prescribing first, minimum, and last flip angles as well as the flip angle corresponding to the center of k-space. A 90° minimum flip angle was used to minimize signal loss due to cardiac pulsation in the left lobe of the liver.
The under-sampled wave-encoded k-space was used to estimate the wave-PSF3. Auto-calibrated estimation of coil sensitivity maps4 (ESPIRiT5), and CS-SENSE image reconstruction6 with $$$\ell$$$1-wavelet regularization were performed in MATLAB and C (the BART toolbox7). Simulated g-factor maps of wave-encoded and non-wave-encoded acquisitions with uniform under-sampling were compared using estimated coil sensitivity maps of a 32-channel torso coil (NeoCoil, Pewaukee, WI) for uniform sampling patterns.
Phantom and volunteer scans were performed with Institutional Review Board approval and informed consent at 3T (GE MR750, Waukesha, WI) using a 32-channel receive-only torso coil (NeoCoil, Pewaukee, WI) with FE along R/L and PE along A/P. Conventional Cartesian acquisitions were performed for comparison using the same sampling pattern and reconstruction framework.
Results
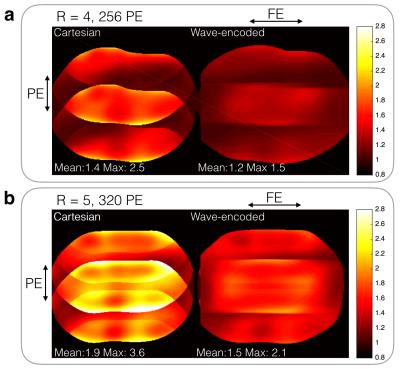
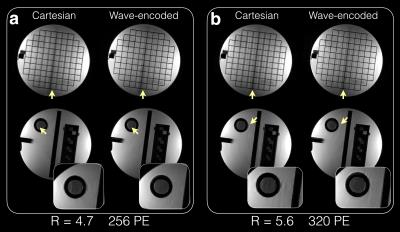
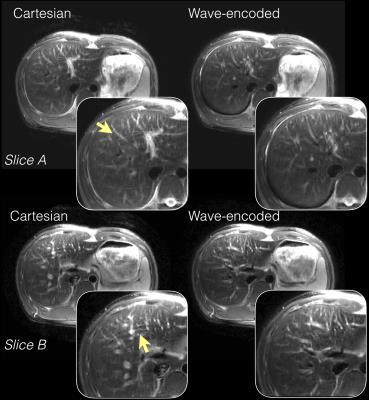
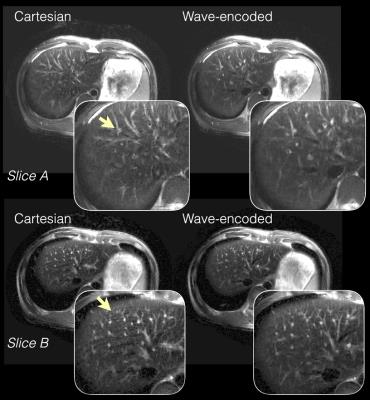
With an acceleration factor of 4 (Fig. 2a), wave encoding achieved an average g-factor of 1.2 and a peak g-factor of 1.5, while the corresponding Cartesian acquisition resulted in a g-factor of 1.4 and a peak g-factor of 2.5. Wave encoding also reduced the average g-factor from 1.9 to 1.5 and reduced the peak g-factor from 3.6 to 2.1 when a 5x acceleration was used (Fig. 2b). Phantom (Fig. 3) and in-vivo images (Fig. 4 and 5) of wave-encoded acquisitions showed reduced residual aliasing artifacts, less blurring, and more structural details than conventional Cartesian acquisitions. Similar T2 contrast was observed in Cartesian and wave-encoded images. The reconstruction time per slice was 10s for Cartesian and 20s for wave encoding, and the calibration of wave-PSF took about 60s prior to reconstruction.Discussion
Wave encoding creates voxel spreading in the FE direction2. Therefore, it makes use of coil sensitivities more efficiently and achieves lower g-factors than Cartesian acquisitions. When incorporated in VD-SSFSE readouts, wave encoding significantly improves the image quality at an effective acceleration factors of about 5. The readout duration, echo-spacing, and TE of wave-encoded acquisitions are within 3 ms of those of conventional Cartesian acquisitions, which helps maintain T2 contrast. Auto-calibration of the wave-PSF and the coil sensitivity maps using the under-sampled wave-encoded k-space is intrinsically more robust to motion, which is critical for body applications, and reduces the complexity of translating the proposed approach to clinical practice.Conclusion
Compared to conventional non-wave-encoded Cartesian imaging, the proposed wave-encoded sampling achieves improved image quality with reduced aliasing artifacts at higher acceleration factors and with full k-space coverage, thus providing fast acquisitions and clinically relevant echo times. Combined with variable-density sampling and compressed sensing, wave-encoded sampling enables acceleration factors of close to 5 for 2D SSFSE scans.Acknowledgements
GE Healthcare, NIH R01 EB009690, NIH R01 EB019241, P41 EB015891.References
[1] Taviani V, Litwiller DV, Tamir JI, Loening AM, Hargreaves BA, and Vasanawala SS. Variable Density Compressed Sensing Single Shot Fast Spin Echo. Proc. Intl. Soc. Mag. Reson. Med. 2016; 24: 618.
[2] Bilgic B, Gagoski BA, Cauley SF, Fan AP, Polimeni JR, Grant PE, Wald LL, Setsompop K. Wave-CAIPI for highly accelerated 3D imaging. Magnetic Resonance in Medicine. 2015; 73: 2152-2162.
[3] Chen F, Zhang T, Cheng JY, Pauly JM, and Vasanawala SS. Auto-Calibrating Wave-CS for Motion-Robust Accelerated MRI. Proc. Intl. Soc. Mag. Reson. Med. 2016; 24: 1857.
[4] Uecker M, Lai P, Murphy MJ, Virtue P, Elad M, Pauly J, Vasanawala SS, Lustig M. ESPIRiT - An Eigenvalue Approach to Autocalibrating Parallel MRI: Where SENSE meets GRAPPA. Magn Reson Med 2014; 71:990-1001.
[5] Curtis A, Bilgic B, Setsompop K, Menon RS, Anand CK. Wave-CS: Combining wave encoding and compressed sensing. Proc. Intl. Soc. Mag. Reson. Med. 2015; 23: 0082.
[6] Cauley SF, Setsompop K, Bilgic B, Bhat H, Gagoski B, Wald LL. Autocalibrated wave-CAIPI reconstruction; Joint optimization of k-space trajectory and parallel imaging reconstruction. Magnetic Resonance in Medicine. 2016; Epub ahead of print.
[7] Uecker M, Ong F, Tamir JI, Bahri D, Virtue P, Cheng JY, Zhang T, Lustig M. Berkeley Advanced Reconstruction Toolbox. Proc. Intl. Soc. Mag. Reson. Med. 2015; 23:2486.
Figures