1035
The Role of the L-type calcium channel Cav1.2 in MEMRI1Max Planck Institute of Psychiatry, Munich, Germany
Synopsis
L-type voltage-dependent Ca2+ channels of type Cav1.2, but not Cav1.3, are essential for Mn2+ accumulation in MEMRI. Cav1.2 conditional knock-out animals were exploited to show that a bias exists towards Mn2+ accumulation in axon terminals and highly dense output structures. Our results have strong implications for the analysis of activity dependent Mn2+ accumulation.
Purpose
Manganese-enhanced MRI1 (MEMRI) exploits the biochemical similarity of Ca2+ and Mn2+ to map the brain’s activity in vivo. To what extend different calcium channels add to the signal increase is not yet understood. Here, we studied the role of L-type voltage-dependent Ca2+ channels (Cav1.2 and Cav1.3) in MEMRI contrast. We further exploit this model to study the fate of Mn2+ accumulation after long-term systemic application.Methods
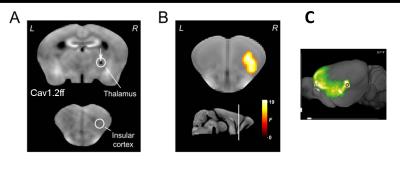
First, genetic deletion of the voltage-dependent channels Cav1.2 or Cav1.3 in the brain was performed as described in Langwieser et al.2 and in Platzer et al.3, respectively, in order to establish a full knock-out model of the respective channel. In a second experiment, homozygous floxed CAv1.2 mice were injected in the thalamus with adeno-associated viruses (AAV) encoding the Cre recombinase (AAV-GFP/Cre) or exclusively green fluorescent protein (AAV-GFP) as a control. AAV-mediated delivery of the Cre recombinase by AAV-GFP/Cre allows a time and region specific knockout of Cav1.2 after injection. Third, a local injection of Mn2+ into the thalamus was performed in one animal to study draining of Mn2+ to projection terminals. For experiments 1 and 2, animals first underwent baseline MRI (7T, 3D-T1w, TR = 50 ms, TE = 3.2 ms, 125x125x140 μm3 resolution) followed by MEMRI experiment as detailed in Grünecker et al.4. After baseline MRI, mice had one week of rest before they received intraperitoneal injections of 30mg/kg manganese chloride every 24 hours for eight consecutive days (8x30/24h)4, followed by the second MEMRI scan. For experiment 3, one animal received a single intracranial injection of Mn2+ in the thalamus, and was repeatedly scanned each 12h using the above mentioned MEMRI experiment.Results
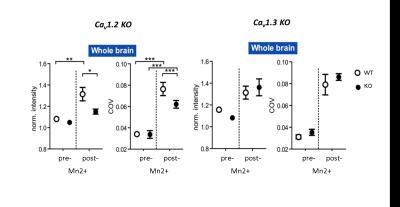
MEMRI intensities in ROIs of the hippocampus, cortex and the whole brain, pre- and post-application of an 8-days fractionated Mn2+ application scheme were assessed. Relative to wild-type animals, MEMRI shows a significantly reduced intensity in all three compartments only for Cav1.2 ko mice, but not for Cav1.3 ko animals (Fig. 1 for whole brain ROI), suggesting an important of Cav1.2 in the transportation of Mn2+. Region specific suppression of Cav1.2 expression exclusively in the thalamus does not lead to reduced accumulation in the primarily targeted brain region after repeated i.p. injection of Mn2+, but in the insular cortex (Fig. 2), which constitutes an axonal terminal of the thalamus. Using repeated scanning over 2 days after a single dose intracerebral delivery of Mn2+ in the thalamus of a wt mouse, we could show that initial accumulation inside the thalamus appears to drain towards the axonal projection terminal.Discussion
Our results highlight a specific role of the voltage-dependent Cav1.2 channel in Mn2+ accumulation. Furthermore, after systemic fractionated application of Mn2+ (i.e. over an extended period of time), axonal projection terminals appear to show increased accumulation as compared with primary uptake structures. This strongly influences interpretation of MEMRI data with respect to altered activation patterns, especially when fractionated schemes or long-term Mn2+ delivery using osmotic mini-pumps is employed.Conclusion:
We describe for the first time the essential role of voltage-dependent calcium transporter Cav1.2 in accumulation of manganese in the living mouse brain. In addition, we highlight the importance of considering intra-cerebral Mn2+ axonal transportation also during systemic Mn2+ delivery routes.Acknowledgements
S.A-C. was supported by a Science Without Borders, CAPES, PhD stipend (BEX 9694-13-7) from BrazilReferences
1. Silva AC, Lee JH, Aoki I, Koretsky AP. Manganese-enhanced magnetic resonance imaging (MEMRI): methodological and practical considerations. NMR Biomed. 2004;17(8):532-543
2. Langwieser N, Christel CJ, Kleppisch T, Hofmann F, Wotjak CT, Moosmang S. Homeostatic switch in hebbian plasticity and fear learning after sustained loss of Cav1.2 calcium channels. J Neurosci. 2010;30(25):8367-8375
3. Platzer J, Engel J, Schrott-Fischer A, et al. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102(1):89-97
4. Grunecker B, Kaltwasser SF, Peterse Y, et al. Fractionated manganese injections: effects on MRI contrast enhancement and physiological measures in C57BL/6 mice. NMR Biomed. 2010;23(8):913-921
Figures