1033
Molecular imaging of inflammation and extracellular matrix remodeling in a murine model of myocardial infarction1Division of Imaging Sciences and Biomedical Engineering, King's College London, London, United Kingdom, 2Cardiovascular Division, The British Heart Foundation Centre of Excellence, King's College London, London, United Kingdom, 3Heinrich Heine University Düsseldorf, Düsseldorf, Germany, 4Cardiovascular Division, James Black Centre, King's College London, London, United Kingdom
Synopsis
A suitable degree and timely resolution of inflammation and extracellular matrix (ECM) deposition are requirements for optimal healing and remodeling after myocardial infarction (MI). In this study, we explored the merits of multinuclear 1H/19F MRI for the simultaneous assessment and quantification of cardiac inflammation and elastin deposition in a murine model of MI. 19F containing particles, uptaken by macrophages, were used to investigate inflammatory cell recruitment into injured myocardium and an elastin-specific MR contrast agent was used to evaluate changes in elastin content in the ECM post-MI.
Purpose
Myocardial infarction (MI) is one of the major health care problems in western societies1. Magnetic resonance imaging (MRI) has great potential for quantification of key biological processes post-MI, such as inflammatory cell recruitment and extracellular matrix (ECM, elastin and collagen deposition) remodeling with the use of novel target specific contrast agents. During the acute phase following MI, the degree and duration of the inflammatory response critically affects myocardial remodeling and cardiac function. 19F perfluorocarbons (PFCs) uptaken by inflammatory cells allow direct detection and quantification of the temporal and spatial evolution of the inflammatory response in the injured myocardium2. During the maturation phase post-MI, the synthesis of elastin, an important ECM protein, is upregulated and can be imaged by MRI using an elastin-specific contrast agent (Gd-ESMA)3. Multinuclear 1H/19F MRI allows both assessment of inflammatory response and ECM remodeling in the healing myocardium and thus allows to investigate the interplay of these biological processes and its effects on cardiac structure and function for future diagnosis and prognosis.Methods
MI was induced in female wild type C57BL/6J mice by permanent ligation of the left anterior descending coronary artery. In vivo MRI. 8 animals were imaged at 3,7,14 and 21days post-MI and 6 mice were imaged longitudinally at 7 and 21 days post-MI using a 3T MR scanner. Animals were scanned using a 1H/19F surface coil (diameter=33mm and 23mm). Anesthesia was maintained with 1-2% isoflurane in oxygen, and body temperature was maintained using a water-based heating system and a rectal temperature feedback probe. Short-axis 1H and 19F ECG-triggered images were acquired after intravenous injection of 0.5mmol/kg of Gd-ESMA and 400μL of 19F-PFCs, 1h and 48h before the scan, respectively. Following a 3D-GRE scout scan, 2D cine short-axis images were acquired covering the entire left ventricle. 80-100min after Gd-ESMA injection, a 2D-Look-Locker sequence was used to identify the optimal inversion time(TI) to null healthy myocardium. 3D late-gadolinium-enhancement(LGE) images were acquired for infarct visualization with the following parameters:FOV=35x35x12mm,in-plane resolution=0.3x0.3x1mm,slices=12,TR/TE=6.4/2.6ms,5 heart beats between subsequent IR pulses, and flip angle=25°. A 3D-GRE sequence preceded by a non-selective inversion pulse was used for T1-mapping The inversion pulse was followed by eight segmented readouts, each spaced one RR-interval apart, for eight individual images resulting in TI’s ranging from 10ms to 2000ms. To allow full magnetization recovery, 12 pause heart beats were performed before the next inversion pulse. Acquisition parameters included:FOV=35x35x1.5mm, in-plane resolution=0.3x0.3mm,slices=1,TR/TE=7.5/3.1ms,flip angle=16°. 3D turbo-spin echo 19F scans were acquired with a FOV=35x35x12mm,in plane resolution=1x1x2mm,slices=12,TR/TE=4beats/8.9ms,TSE factor=5,offset frequency=10200Hz(BW=6103Hz). A saturation slice was used to suppress liver signal. To enable SNR calculation, a noise-scan was acquired with the same imaging parameters but without any RF pulses. Histology. Immunohistochemistry(IHC) was performed to quantify tropoelastin (non-crosslinked elastin) and macrophage content.Results and Discussion
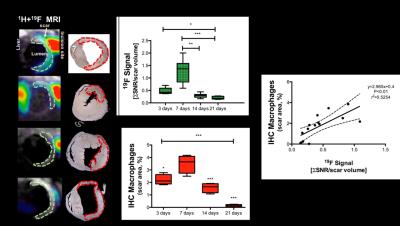
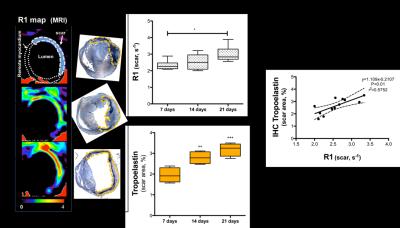
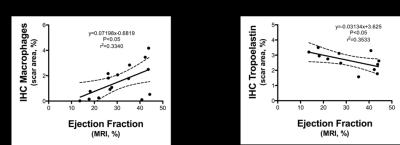
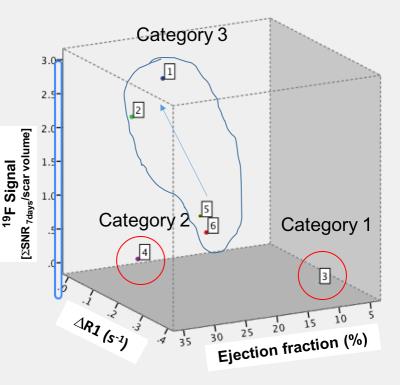
Acquisition of morphologically matching proton (1H) and fluorine (19F) images enables anatomical location of the PFCs. A significant increase of 19F signal within the infarcted area was detected at 3 and 7 days post-MI (Fig.1A&1B) and correlated with an increase in macrophage content as evaluated by IHC (Fig.1C&1D). R1 maps, after Gd-ESMA administration showed deposition of elastin in the infarcted area from 7 days post-MI onwards (Fig.2A&2B). This was in agreement with the deposition of tropoelastin (Fig.2C&2D) as measured with IHC. The interplay between inflammation, elastin remodeling and cardiac function was also investigated. Correlation analysis showed a linear correlation between inflammation and ejection fraction (r=0.58 P<0.05, Fig.3A) whereas a negative correlation was observed between tropoelastin and cardiac function (r=-0.60 P<0.05, Fig.3B). The potential prognostic value of these measurements was evaluated in a longitudinal study (Fig.4). Remodeling post-MI is a dynamic and complex process where several factors can influence cardiac outcome after MI (e.g.infarct size, degree and duration of inflammation and ECM deposition). Our longitudinal data suggest that when studying the interplay of inflammation, elastin remodeling and recovery of the ejection fraction over time the animals could be clustered in three categories: (1) those with the worst outcome (lower EF) had an extremely low inflammatory response and high tropoelastin content (19F<<Gd-ESMA), (2) those with the best outcome (higher EF) had an inflammatory response that was balanced by tropoelastin production (19F=Gd-ESMA), and (3) those with an intermediate response (EF values inbetween) had an inflammatory response that was higher than the tropoelastin production (19F>>Gd-ESMA).Conclusions
Our results demonstrate not only the feasibility of multinuclear 1H/19F MRI for the simultaneous assessment of inflammation and elastin remodeling following myocardial infarction in a murine model but also the interplay of these biological processes and their effects on cardiac outcome which may have potential for improved diagnosis and prognosis.Acknowledgements
British Heart Foundation PhD studentship and British Heart Foundation Program grant (RG/12/1/29262).References
1. WHO. Cardiovascular disease, 2016.
2. Flogel U, et al. Circulation, 2008;118(2):140-148.
3. Wildgruber M, et al. Circ Cardiovasc Imaging, 2014;7:321-329.
Figures