1032
Molecular MR imaging of pulmonary fibrogenesis using the novel probe GdOA1A.A. Martinos Center for Biomedical Imaging, Charlestown, MA, United States, 2Center for Immunology and Inflammatory Diseases, Massachusetts General Hospital, Charlestown, MA, United States
Synopsis
Fibrogenesis is a feature of idiopathic pulmonary fibrosis (IPF) that leads to the increased deposition and cross-linking of collagen. There remains a demand for non-invasive imaging of fibrogenesis in patients with suspected IPF to deliver earlier diagnoses and monitor treatment response. One universal feature of fibrogenesis is the oxidation of lysines on collagen to form allysine, which is a fundamental component for the cross-linking of collagen. We developed GdOA, a Gd-based MR probe that targets allysine as a marker for active fibrogenesis. We demonstrate that GdOA MR signal enhancement correlates with extent of disease and is sensitive to therapeutic response.
Introduction
Universal diagnosis of idiopathic pulmonary fibrosis (IPF)1 based on high-resolution-CT assessment alone remains a challenge, and while biopsy provides a gold standard of assessment,2 it is invasive and suboptimal for repeat measurement. As such there is a need to deliver non-invasive imaging of IPF for early disease identification. A key component of active fibrosis is allysine, a product of the oxidation of collagen by lysyl oxidase (LOX). Allysine is fundamental to the crosslinking of collagen and presents a suitable biomarker for quantifying fibrogenesis.Purpose
The aim of this study was to a) develop a novel Gd-based molecular MR probe, termed ‘GdOA’ for targeted imaging of allysine, b) validate the selectivity of GdOA binding and c) determine whether GdOA could quantify the extent of fibrogenesis in a mouse model of pulmonary fibrosis.Methods
GdOA was prepared in 7 steps from cyclen alongside GdOX, a non-binding negative control probe. To assess the aldehyde binding potential of GdOA, T1 relaxivity studies were performed, with GdOA and GdOX (10-500 µM) incubated with either BSA or oxidized BSA, termed BSA-Ald (16 nmol aldehyde/mg protein), for 24h (37 °C, pH 7.4). To assess allysine binding, GdOA and GdOX were incubated with allysine rich porcine aorta for 24 h at 37 °C, pH 7.4, and the extent of binding quantified by ICP analysis. To evaluate GdOA in an animal model of pulmonary fibrosis, three cohorts of mice were studied: Group A) sham-treated, Group B) bleomycin-treated (1.0 U/Kg intratracheal) and Group C) bleomycin-treated (1.0 U/Kg) followed by daily injection of the pan-LOX inhibitor BAPN. Animals were imaged with GdOA or GdOX at day 14 post bleomycin injection using an ultrashort echo time sequence before and 12 minutes after injection of probe. The lung-to-muscle ratio (ΔLMR) of lung signal relative to adjacent skeletal muscle was measured pre- and post-injection of probe. Following MRI, lungs were collected and assessed for LOX enzyme activity, allysine concentration and Gd content.Results
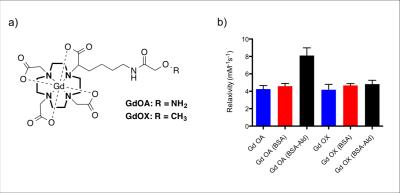
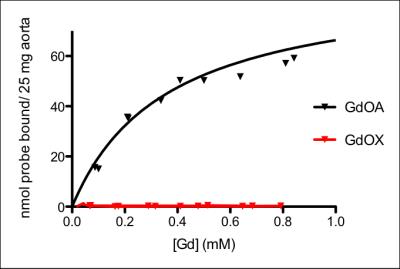
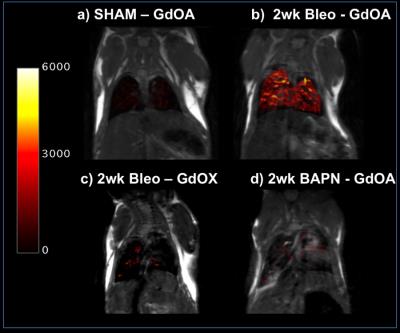
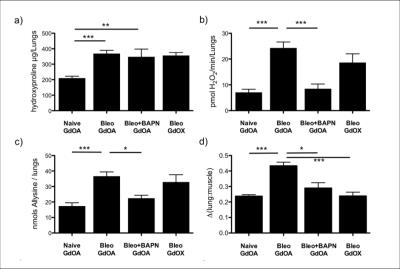
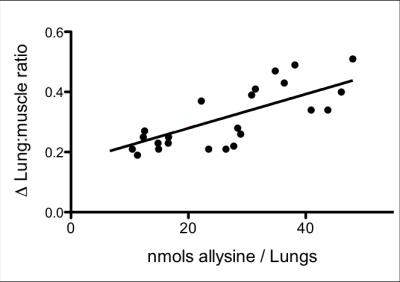
GdOA and GdOX (Figure 1a) were prepared in high purity as assessed by HPLC-ICP analysis. The relaxivity of GdOA was unchanged in the presense of BSA, but increased by 90% when incubated with BSA-Ald (Figure 1b). The relaxivity of GdOX was unchanged in the presence of BSA or BSA-Ald. GdOA gave a Kd of 360 μM for binding to allysine rich aorta (7.5 μmol allysine/g aorta) while GdOX showed no affinity (Figure 2). In a bleomycin mouse model of pulmonary fibrosis, GdOA resulted in an increased ΔLMR in the bleomycin group (0.43±0.02) compared to sham-treated mice (0.24±0.01) (p<0.001), consistant with the increased GdOA uptake observed in fibrotic lung (Figure 3, 4d). No signal enhancement was seen with GdOX. BAPN treatment resulted in GdOA uptake that was significantly decreased compared to the bleomycin group (ΔLMR: 0.25±0.03, p=0.0065) (Figure 4d). Hydroxyproline levels, as a measure of collagen burden, were significantly elevated in the bleomycin-treated animals compared to sham-treated group (p<0.001) (Figure 4a). The collagen burden remained elevated in the BAPN group. LOX enzyme activity was 3.4-fold higher for the bleomycin group compared to sham animals (p<0.001), with the BAPN group showing a 2.9-fold reduction compared to bleomycin-treated mice (p=0.0016) (Figure 4b). A 2.1-fold increase in allysine concentration was observed for the bleomycin group compared to sham-treated mice (p<0.001). Allysine levels in the BAPN group were close to sham levels (Figure 4c). Increases in ΔLMR correlated with increasing concentration of allysine (r = 0.69) (Figure 5).Discussion
GdOA is an oxyamine derivative of GdDOTA designed for targeted binding to allysine, with minimal off-target accumulation and rapid renal excretion. GdOA binds to allysine containing proteins in vitro, but does not bind nonspecifically to BSA. A challenge of molecular MR is having a target at sufficient concentration to be detectable. The allysine concentration in tissue, assessed by HPLC analysis, ranged from 60 nmol/g in sham-treated animals to 150 nmol/g (high micromolar range) in bleomycin-treated mice making it compatible for targeted imaging with MRI. GdOA enhanced MR resulted in strong lung signal enhancement in bleomycin injured lungs compared to lungs of sham-treated mice. Negative control GdOX showed no significant enhancement in bleomycin injured lungs. GdOA lung imaging correlated with lung LOX activity and allysine levels. Treatment with the LOX inhibitor BAPN diminished total LOX and allysine activity and resulted in a subsequently reduced GdOA enhanced MR signal, further demonstrating specificity of the probe for allysine and showing that GdOA can image treatment response.Conclusion
GdOA is a novel molecular MR probe that is highly specific for allysine residues in oxidized collagen, and represents a new method to noninvasively assess pulmonary fibrogenesis.Acknowledgements
This work was supported by NIDDK (DK104302, DK104956), NIBIB (EB009062), NHLBI (HL116315) and the Athinoula A. Martinos Center for Biomedical ImagingReferences
1. J. A. Bjoraker et. al., Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 1998; 157: 199.
2. C. A. Souza et. al., Idiopathic pulmonary fibrosis: spectrum of high-resolution CT findings. AJR Am. J. Roentgenol. 2005;185: 1531.
Figures