1019
A novel fat and iron quantification technique with non-rigid motion-corrected averaging based on non-local means1Radiology, University of Wisconsin-Madison, Madison, WI, United States, 2Electronic engineering, Tsinghua University, Beijing, People's Republic of China, 3Global MR Applications and Workflow, GE Healthcare, Madison, WI, United States, 4Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 5Biomedical engineering, University of Wisconsin-Madison, Madison, WI, United States, 6Medicine, University of Wisconsin-Madison, Madison, WI, United States, 7Emergency Medicine, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
In this study, we developed and validated a free-breathing fat and R2* quantification technique using a multi-average 2D sequential acquisition with non-rigid motion-corrected averaging based on a non-local means (NLM) approach. The proposed technique was applied to simulated data as well as free-breathing liver acquisitions in volunteers. Both direct averaging and the proposed NLM technique were applied to the data to improve SNR. Compared to direct averaging, the proposed NLM technique resulted in improved image quality without motion artifacts, as well as accurate fat and R2* measurements in both simulations and in vivo acquisitions.
Purpose
Chemical shift-encoded MRI (CSE-MRI) techniques provide accurate quantification of proton density fat-fraction (PDFF) 1, and R2* (a biomarker of iron concentration) 2. CSE methods typically use breath-held 3D or 2D slice-interleaved acquisitions to avoid motion artifacts.
A recently proposed 2D sequential CSE-MRI technique, where slices are acquired sequentially with a short temporal footprint, enables motion-robust CSE-MRI of the abdomen3. Unfortunately, this technique suffers from low signal-to-noise ratio (SNR). Acquiring multiple averages of this 2D sequential acquisition during free-breathing to improve its SNR would be highly desirable, however direct averaging may introduce motion-related artifacts. Further, non-rigid 3D motion makes explicit registration of these acquisitions prior to averaging challenging. Therefore, the purpose of this work was to develop a non-local means (NLM) approach for motion-corrected averaging of free-breathing, multi-average 2D sequential CSE-MRI.
Method
Proposed algorithm:
In this work, NLM-based4 motion-corrected averaging from multiple repetitions was performed jointly for all acquired echo times. For each slice, one of the multiple acquired repetitions was manually selected as a reference. For the pixel located at position p for the current slice in the multi-echo reference images ($$$s_{ref}$$$), the algorithm finds the most similar pixel in each of the different slices and repetitions based on neighborhood similarity. The neighborhood similarity is measured by the weighted Euclidean distance$$$||s_{ref}(N_{p}) – s_{i,j}(N_{q})||^{2}_{2,a}$$$, where $$$s_{i,j}(N_{p})$$$ denotes the measured signal for slice i and repetition j, over a neighborhood $$$N_{p}$$$ (which covers a square neighborhood around pixel p, over all acquired echo times). The parameter a denotes the standard deviation of a Gaussian weighting on the weighted Euclidean distance, placing larger weight on pixels closer to the center of the square neighborhood. For a pixel p in the reference repetition, the most similar pixel’s position for slice i and repetition j is denoted by $$$q_{p,i,j}$$$. Subsequently, the output pixel values at location p for the current slice are calculated as
$$v_{out}(p) = \sum_{i,j} w(p,q_{p,i,j})v_{in}(q_{p,i,j})$$
The weight $$$w(p,q_{p,i,j})$$$ is:
$$w(p,q_{p,i,j})=\frac{1}{z}e^{\frac{||s_{ref}(N_{p}) – s_{i,j}(N_{q})||^{2}_{2,a}}{h^{2}}}$$
where z is a normalizing factor and h is a tunable parameter that balances motion robustness and SNR.
Parametric mapping:
For both simulated and in vivo datasets (see below), PDFF and R2* of the outputs of NLM, direct averaging as well as from the original single-average noisy images5,6 were calculated.
Simulations:
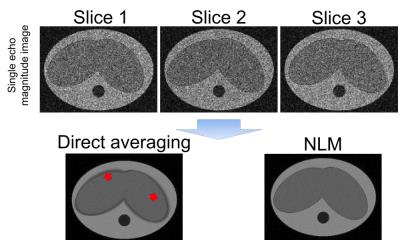
A simple 3D model of abdomen was generated (see Figure 1). In this model, the liver undergoes elastic, non-rigid, motion in the axial plane and translation along the S/I direction. The simulated respiratory rate was 10 breaths/minute. Simulated acquisition parameters included: 7mm slices, matrix size=144×144, 8 TEs, TE1=1.2ms, DTE=1.4ms and SNR=20.
Volunteers:
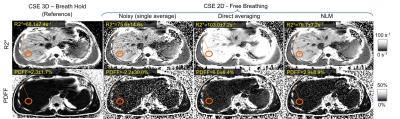
Three volunteers were scanned prospectively at 3T (MR750, GE Healthcare, Waukesha, WI) using a 32-channel phased array torso coil (Neocoil, Pewaukee, WI). Reference PDFF and R2* quantification was performed using a breath-held 3D CSE-MRI sequence with a standard protocol7: FA=3°, FOV=40×30×26cm3, matrix=224×160×32, 8mm slices, 6TEs, bandwidth=±125kHz, TR=9.5ms, TE1=1.1ms, DTE=0.9ms. Additionally, 2D sequential CSE-MRI data were acquired during free-breathing with 10 averages. Typical parameters include: flip angle=3°, FOV=40×30cm2, matrix=192×144 , 8mm slices, 6TEs, bandwidth=±62.5kHz, TR=9.5ms, TE1=1.7ms, DTE=1.2ms.
Results
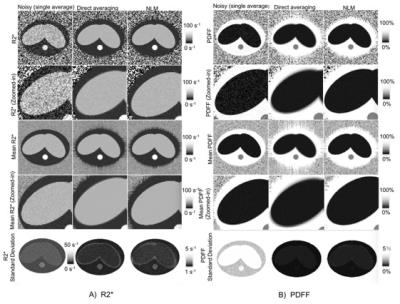
Simulation results are shown in figures 1 and 2. As shown in the second row of Figure 1, the output of NLM has similar (high) SNR as direct averaging, but NLM results in reduced artifacts and blurring. This effect is also observed in the PDFF and R2* maps (Figure 2).
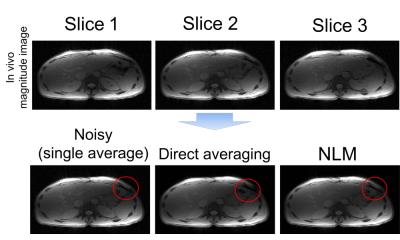
Figure 3 illustrates processing of in vivo liver data. Direct averaging introduces artifacts near tissue interfaces in the presence of motion (see red circles). Regions-of-interest (ROIs) (Figure 4-5) were placed in R2* and PDFF of images of reference, single average, direct averaging and NLM, in order to measure R2* and PDFF in the right lobe of the liver. The standard deviation within the ROI were reduced by both direct averaging and NLM compared to the noisy single-average images. NLM resulted in improved accuracy of PDFF and R2* compared to direct averaging.
Discussion and Conclusion
In this work, we have demonstrated the feasibility and potential effectiveness of a novel NLM-based approach for multi-average free-breathing PDFF and R2* quantification in the abdomen. This technique enables motion-corrected averaging without the need for explicit non-rigid registration. In this preliminary study, the feasibility of the proposed technique was evaluated in healthy subjects. Further quantitative validation in patients, particularly children and those patients unable to hold their breath, is needed.
In conclusion, the proposed 2D sequential technique with NLM-based motion-corrected averaging may enable accurate quantification of fat and iron in the liver with high SNR and excellent robustness to motion.
Acknowledgements
The authors wish to acknowledge support from the NIH (UL1TR00427, R01-DK083380, R01-DK088925, R01-DK100651, K24-DK102595), as well as research support from GE Healthcare.References
1.Reeder, Scott B.,et al. "Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy". Journal of magnetic resonance imaging 34.4 (2011): 729-749.
2.Wood, John C., et al. "MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients". Blood 106.4 (2005): 1460-1465.
3. Ruby et al, "Motion Intensive Quantitation of Liver Proton Density Fat-fraction Using a Single-Shot 2D Technique", ISMRM 2016.
4.Buades, Antoni, Bartomeu Coll, and J-M. Morel. "A non-local algorithm for image denoising". 2005 IEEE Computer Society Conference on Computer Vision and Pattern Recognition (CVPR'05). Vol. 2. IEEE, 2005.
5.Yu, Huanzhou,et al. "Multiecho water-fat separation and simultaneous R 2* estimation with multifrequency fat spectrum modeling". Magnetic resonance in medicine 60.5 (2008): 1122-1134.
6.Hernando, Diego, J. Harald Kramer, and Scott B. Reeder. "Multipeak fat-corrected complex R2* relaxometry: Theory, optimization, and clinical validation". Magnetic resonance in medicine 70.5 (2013): 1319-1331.
7.Reeder, Scott B., et al. "Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy." Journal of magnetic resonance imaging 34.4 (2011): 729-749.
Figures