1017
T1-weighted two-point fat/water separated PROPELLER acquired with dual bandwidths1Neuroradiology, Karolinska University Hospital, Stockholm, Sweden, 2Department of Clinical Science, Intervention and Technology, Karolinska Institutet, Stockholm, Sweden, 3Department of Medical Radiation Physics, Karolinska University Hospital, Stockholm, Sweden, 4Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden
Synopsis
A PROPELLER sequence with dual bandwidths was developed for T1-weighted fat/water separated imaging. The bandwidth of the second readout was adjusted to remove dead time related to shifted readouts in order to improve SNR efficiency. Before PROPELLER reconstruction, bladewise fat/water separation was performed in k-space to remove chemical shift displacement artifacts. This enabled low bandwidth acquisitions without smearing of the fat signal, which further improved SNR efficiency. Strong fat suppression insensitive to B0 inhomogeneity was demonstrated in imaging of the neck and orbits.
Purpose
The PROPELLER technique (1) is an alternative to the fast spin-echo sequence with a sampling scheme more robust to motion. Combined with retrospective motion correction and rejection, it is possible to further reduce motion artifacts. With long echo train lengths (ETL), it is difficult to achieve T1 weighted images because of magnetization transfer effects. Therefore, alternative approaches have been presented where ETL is short (2). As the displacement of fat due to chemical shift varies between blades, the fat signal appears smeared in PROPELLER images. This effect can be minimized by sampling with a high receiver bandwidth, at the cost of reduced SNR efficiency. Spectral fat saturation can also be used, but is sensitive to B1 and B0 inhomogeneity, which becomes increasingly problematic at higher field strengths.
As an alternative, chemical shift based fat/water separation offers strong and uniform fat suppression. Moreover, chemical shift displacement artifacts can be removed by performing the separation in k-space (3). Previous work on fat/water separated PROPELLER sequences have focused on T2 weighted (4–7), or T1 FLAIR (7) imaging.
We propose a T1-weighted fat/water separated PROPELLER sequence with dual bandwidths that maximizes SNR efficiency by removing any dead time related to shifted readouts.
Methods
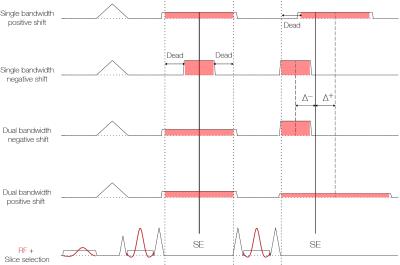
An in-house developed PROPELLER sequence was modified so that each shot contains two refocusing pulses. The second echo from each shot was shifted relative to the spin echo. The receiver bandwidth of the second readout was adjusted to sample the k-space center at the desired echo shift without any dead time, as shown in Figure 1. In addition to contributing with spectral information enabling fat/water separation, the second echo also increases SNR in the resulting images.
A 3 T clinical MRI system (DVMR750, GE Healthcare, Milwaukee, WI, USA) with an 8-channel head-neck receive coil was used to acquire neck and orbit datasets from two healthy subjects who gave informed consent. Imaging parameters for neck/orbits were: FOV = 20/26 cm, 20/14 blades with a 512x64 matrix, slice thickness = 4/3 mm, TR = 500 ms, TE1 = 16 ms (rBW1 = ±31 kHz), TE2 = 31/29 ms (rBW2 = ±25/42 kHz) shifted 1.0 ms after/before the spin echo.
After coil combination, fat/water separation was performed bladewise prior to the PROPELLER reconstruction. A multi-scale graph-cut based algorithm (8) was used for B0 correction before water and fat were separated in k-space, taking the multiple resonances of fat into account (9). Similar to previous methods, central k-space samples are acquired at the desired echo shift, while peripheral samples deviate from this shift. This may lead to ill-conditioning of the inverse problem, which is further complicated with the dual bandwidth design. We improved the conditioning with Thikonov regularisation (10) where the Thikonov factor at each k-space point was chosen to achieve a certain maximum condition number.
SNR efficiency, defined as the SNR over the square root of the scan time, is proportional to the square root of the fraction of scan time spent on signal readout. For each acquisition, the theoretical improvement in SNR efficiency was calculated compared the corresponding single bandwidth case with associated dead time (Figure 1).
Results
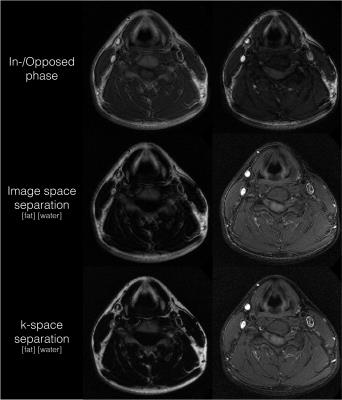
Figure 2 shows the proposed method with the fat/water separation performed in image- and k-space, respectively. It is evident that k-space separation is able to avoid smearing of the fat signal.
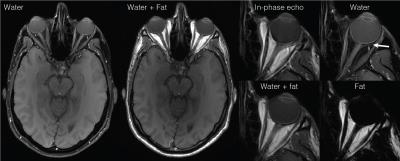
Figure 3 shows water- and fat only images of the orbits. Chemical shift displacements are removed and T1 contrast is maintained. The fat signal is strongly suppressed in the water only image despite the proximity to the nasal sinuses where susceptibility effects are large.
For the neck/orbits acquisitions, the dual bandwidth approach improved the SNR efficiency by 3.1/8.0%.
Discussion & Conclusion
The chemical shift displacement will vary both between blades and between echoes with the proposed dual-bandwidth approach. The k-space based water/fat separation compensates for these effects by taking into account the exact time shift of each sample, rendering water and fat images free from displacement artifacts such as fat smearing (Figure 2). This allows low bandwidth acquisitions, which increases SNR efficiency. The removal of dead time improves SNR efficiency further.
In conclusion, we present a two-point fat/water separated T1-weighted PROPELLER sequence that removes dead time and allows for lower receiver bandwidth, resulting in a higher SNR efficiency. The sequence offers T1-weighted images robust to motion with strong and homogeneous fat suppression.
Acknowledgements
No acknowledgement found.References
1. Pipe JG. Motion correction with PROPELLER MRI: application to head motion and free-breathing cardiac imaging. Magn. Reson. Med. 1999;42:963–969.
2. Skare S, Lilja A. Spin-Echo Propeller (SE-prop): T1-w single-echo motion robust imaging without inversion pulses. In: ; 2006. pp. 1298–1307.
3. Brodsky EK, Holmes JH, Yu H, Reeder SB. Generalized k-space decomposition with chemical shift correction for non-Cartesian water-fat imaging. Magn. Reson. Med. 2008;59:1151–1164.
4. Weng D, Pan Y, Zhong X, Zhuo Y. Water–fat separation with parallel imaging based on BLADE. Magn. Reson. Imaging 2013/6;31:656–663.
5. Schär M, Eggers H, Zwart NR, Chang Y, Bakhru A, Pipe JG. Dixon water-fat separation in PROPELLER MRI acquired with two interleaved echoes. Magn. Reson. Med. 2016;75:718–728.
6. He Q, Weng D, Zhou X, Ni C. Regularized iterative reconstruction for undersampled BLADE and its applications in three-point Dixon water–fat separation. Magn. Reson. Med. 2011;65:1314–1325.
7. Huo D, Li Z, Aboussouan E, Karis JP, Pipe JG. Turboprop IDEAL: A motion-resistant fat–water separation technique. Magn. Reson. Med. 2009;61:188–195.
8. Berglund J, Skorpil M. Multi-scale graph-cut algorithm for efficient water-fat separation. Magn. Reson. Med. [Internet] 2016. doi: 10.1002/mrm.26479.
9. Yu H, Shimakawa A, McKenzie CA, Brodsky E, Brittain JH, Reeder SB. Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn. Reson. Med. 2008;60:1122–1134.
10. Lu W, Yu H, Shimakawa A, Alley M, Reeder SB, Hargreaves BA. Water-fat separation with bipolar multiecho sequences. Magn. Reson. Med. 2008;60:198–209.
Figures