1016
Multi-Parametric/-Nuclear 1H/23Na Clinical Protocol of the Prostate at 3T using a Double Resonant Coil1Computer Assisted Clinical Medicine, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany, 2Institute of Clinical Radiology and Nuclear Medicine, University Medical Center Mannheim, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany
Synopsis
Prostate cancer is the most common malignancy in men. T2-weighted magnetic resonance imaging (MRI) has been shown to provide detailed information about anatomical structures but suffers from low specificity of morphological abnormalities. To enhance identification of malignant lesions, we set up a multi-parametric and multi-nuclear clinical protocol, in which sodium (23Na-) MRI and diffusion weighted imaging with standard and high b-values are added to the morphological T2-weighted sequences. Using a double resonant 23Na/1H abdominal coil allows acquiring sodium images directly after high resolution clinical proton sequences. Coil changes become unnecessary, which improves clinical feasibility.
Purpose
For patients with suspected prostate cancer, T2-weighted MR images are essential diagnostic examinations. They provide detailed information about anatomical structures of the prostate to assess morphological abnormalities. However, the specificity is relatively low1. Prostate cancer is either depicted as a circumscribed low-signal-intensity lesion in the peripheral gland, or as an ill-defined lesion with obscured margins in the transitional zone ("erased charcoal sign"). Yet, post-biopsy bleeding, prostatitis, scarring, or hyperplastic nodules may have a similar T2-appearance2, and differentiation on morphological sequences may be impossible.
To increase specificity of the examination, using a multi-parametric MR protocol of the prostate is widely accepted and multiple studies have been published to confirm the additional value of diffusion-weighted imaging (DWI) and dynamic contrast-enhanced (DCE) MRI3,4. DWI has advanced to a standard diagnostic tool to characterize prostate cancer5 and ultra-high b-value images have been confirmed beneficial for cancer detection6.
Recently, quantitative sodium (23Na-) MRI has been proposed as a possible additional imaging biomarker to increase specificity and diagnostic confidence of the prostate examination7,8,9 as it provides a non-invasive quantitative measure of the tissue sodium concentration (TSC), which might be useful for both cancer treatment response assessment and tumor detection10. Yet, this technique is still a research tool, also due to hardware constraints11.
By using a double resonant 1H/23Na coil with sixteen 23Na receive arrays, we envision overcoming such constraints towards a state-of-the-art multi-parametric but also multi-nuclear prostate exam. It allows precise registration between 1H and 23Na measurements, more sodium signal-to-noise (SNR) in the prostate and saves the examination time since coil changes become unnecessary. Here, we present first measurements on healthy volunteers to establish a reference combining 23Na-MRI and DWI with high b-values.
Methods
All measurements were performed on a 3T clinical whole-body MR scanner (Skyra MAGNETOM, Siemens Medical Solutions, Germany) using a double resonant 23Na/1H coil (RAPID Biomedical GmbH, Germany). Sodium RF transmission is applied by one channel split in four elements, which enables a quadrupole transmission, and receiving is performed by sixteen surface coils. Proton receiving is realized by six arrays and the transmission is performed by the system’s body coil. Nine healthy male volunteers (28 ± 4 years) were scanned with the multi-parametric/-nuclear MR protocol. Standardized 0.45% NaCl solution without agarose and 0.45% and 0.90% NaCl solutions including 2% agarose were placed inside the field-of-view (FoV) to serve as calibration vials. Therefore, sodium signal intensities can be quantified as sodium concentrations. The multi-parametric/-nuclear MR protocol included the following sequences:
· Coronal, sagittal and transversal T2-weighted turbo spin echo sequences: TR/TE/alpha$$$\,$$$=$$$\,$$$3350$$$\,$$$ms/108$$$\,$$$ms/160°, FoV 250x242$$$\,$$$mm2, resolution 0.7x0.7$$$\,$$$mm2, slice thickness 3$$$\,$$$mm, 23 slices, GRAPPA acceleration factor 2, TA$$$\,$$$=$$$\,$$$2:49$$$\,$$$min for each direction
· Two transversal DWI echo-planar imaging sequences: TR/TE$$$\,$$$=$$$\,$$$4100$$$\,$$$ms/74$$$\,$$$ms, FoV 380x80$$$\,$$$mm2, resolution 1.9x1.9$$$\,$$$mm2, slice thickness 3$$$\,$$$mm, 23 slices, b-values: 0/50/400/800$$$\,$$$s/mm2, TA$$$\,$$$=$$$\,$$$2:24$$$\,$$$min and TR/TE$$$\,$$$=$$$\,$$$4900$$$\,$$$ms/96$$$\,$$$ms, b-value: 2000$$$\,$$$s/mm2, TA$$$\,$$$=$$$\,$$$2:42$$$\,$$$min
· Sodium 3D density adapted radial UTE12: TR/TE/alpha$$$\,$$$=$$$\,$$$120$$$\,$$$ms/0.55$$$\,$$$ms/85°, FoV (320$$$\,$$$mm)3, resolution (5$$$\,$$$mm)3, readout length per spoke$$$\,$$$=$$$\,$$$20$$$\,$$$ms, projections$$$\,$$$=$$$\,$$$8000, TA$$$\,$$$=$$$\,$$$16:00$$$\,$$$min.
Sodium reconstruction was done offline in MATLAB (Mathworks, Natick, MA) with a convolution-based regridding algorithm (Kaiser Bessel, width$$$\,$$$=$$$\,$$$4) and application of a Hanning filter in k-space. Combinations of array-images from different coils were performed using adaptive combination13. Sensitivity profiles from the channels were corrected in post-processing14. ADC maps were calculated inline by the scanner software using the first DWI measurement.
Results
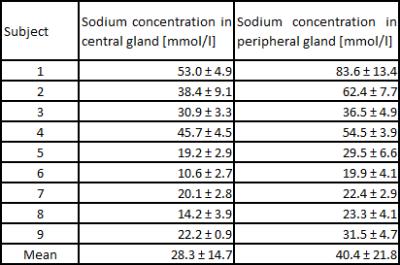
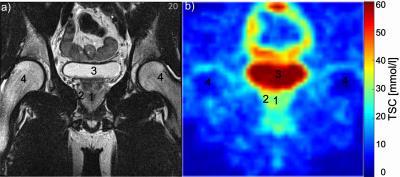
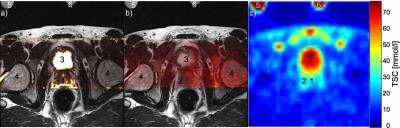
Sodium concentrations were evaluated in the central and peripheral gland (Figure 1). Concentrations were higher in the peripheral (mean 40.4 ± 21.8 mmol/l) than in the central gland (mean 28.3 ± 14.7 mmol/l) in each volunteer. The prostate and its different compartments were identifiable in all volunteers (Figure 2). In Figure 3, an ADC map and a high b2000 superimposed to the T2-weighted image are displayed together with quantified sodium. As expected, b2000 showed no signal in the healthy volunteers.Discussion and Conclusion
We successfully implemented a multi-parametric/-nuclear 23Na/1H clinical protocol of the prostate at 3T using a double resonant coil with high SNR surface coils. Evaluations of ADC, b2000, and TSC were performed in nine healthy volunteers. TSC values show the same trend as previously reported in Hausmann et al.7. In contrast to Peterson et al.9, we applied a UTE sequence for sodium imaging considering the short bi-exponential T2 decay of 23Na. The next step is applying the protocol to patients and adding DCE-MRI. B2000 images will be used as a screening tool for the depiction of malignant tumors. By adding TSC measurements to the protocol, we expect a higher specificity to identify and grade prostate cancer.Acknowledgements
This research project is part of the Research Campus M²OLIE and funded by the German Federal Ministry of Education and Research (BMBF) within the framework “Forschungscampus: public-private partnership for Innovations” under the funding code 13GW0092D.References
[1] Futterer JJ, et al. Prostate cancer localization with dynamic contrast-enhanced MR imaging and proton MR spectroscopic imaging. Radiology. 2006;241:449-458.
[2] Turkbey B, et al. Prostate cancer: value of multiparametric MR imaging at 3 T for detection - histopathologic correlation. Radiology. 2010;255:89-99.
[3] Langer DL, et al. Prostate cancer detection with multi-parametric MRI: Logistic regression analysis of quantitative T2, diffusion-weighted imaging, and dynamic contrast-enhanced MRI. J Magn Reson Imaging, 2009;30(2): 327-334.
[4] Kitajima K, et al. Prostate cancer detection with 3 T MRI: Comparison of diffusion-weighted imaging and dynamic contrast-enhanced MRI in combination with T2-weighted imaging. J Magn Reson Imaging, 2010;31(3): 625-631.
[5] Somford DM, et al. Initial experience with identifying high-grade prostate cancer using diffusion-weighted MR imaging (DWI) in patients with a Gleason score G/= 3 + 3 = 6 upon schematic TRUS-guided biopsy: a radical prostatectomy correlated series. Invest Radiol, 2012;47:153-158.
[6] Kang Y, et al. Gliomas: histogram analysis of apparent diffusion coefficient maps with standard-or high-b-value diffusion-weighted MR imaging—correlation with tumor grade. Radiology, 2011;261(3): 882-890.
[7] Hausmann D, et al. Apparent diffusion coefficient and sodium concentration measurements in human prostate tissue via hydrogen-1 and sodium-23 magnetic resonance imaging in a clinical setting at 3 T. Invest Radiol, 2012;47(12): 677-682.
[8] Hausmann D, et al. Sodium imaging of the prostate at 3 T. Proc ISMRM, 2012;20:546.
[9] Peterson JC, et al. In Vivo Sodium Imaging of Human Prostate Cancer. Proc ISMRM, 2015;23:1162.
[10] Jacobs MA et al. Breast Cancer Res Treat, 2011;128(1):119-26.
[11] Zöllner F, et al. Quantitative Sodium MRI of Kidney. NMR Biomed, 2016;29(2):197-205.
[12] Nagel AM, et al. Sodium MRI using a density-adapted 3D radial acquisition technique. Magn Reson Med, 2009;62:1565-1573.
[13] Walsh DO, et.al. Adaptive reconstruction of phased array MR imagery. Magn Reson Med, 2000;43(5):682-690.
[14] Murakami JW, Hayes CE, and Weinberger E, Intensity correction of phased-array surface coil images. Magn Reson Med, 1996;35(4):585–590.
Figures