1009
Microstructural Diffusion-Weighted (VERDICT) MRI Metrics are Repeatable and Show Potential at Characterising Gleason 7 Prostate Cancer Non-Invasively1Centre for Medical Imaging, University College London, London, United Kingdom, 2Centre for Medical Image Computing, University College London, London, United Kingdom, 3Research Department for Tissue & Energy, University College London, London, United Kingdom, 4Department of Pathology, University College London Hospital, University Street, United Kingdom, 5Radiology, University College London Hospitals, London, United Kingdom, 6Department of Urology, University College London Hospital, London, United Kingdom
Synopsis
In this study we we assess the repeatability of VERDICT (Vascular, Extracellular, and Restricted Diffusion for Cytometry in Tumours) MRI parameters in prostate cancer consider their ability to distinguish between Gleason grades compared with the standard ADC model in 71 patients. Four of the parametric maps derived from the VERDICT technique were found to be satisfactorily repeatable for use as a clinical tool, and are capable of identifying a Gleason 7 component in prostate cancer where ADC failed to do so. VERDICT therefore holds great potential for use in clinical prostate cancer management pathways in the future.
PURPOSE:
VERDICT (Vascular, Extracellular, and Restricted Diffusion for Cytometry in Tumours) is a quantitative microstructural imaging technique that combines a diffusion-MRI acquisition with a mathematical model with a view to improved non-invasive cancer characterisation. Demonstrating promise in the preclinical setting1 and in a pilot study in prostate cancer2, as per the imaging biomarker roadmap3 we aim to establish the repeatability of VERDICT parameters, then demonstrate their putative clinical value as classifiers of Gleason grade in patients recruited into the INNOVATE clinical trial4.
We are specifically interested in using the technique to improve specificity for Gleason 7 disease, due to different genomic signatures 5, metastatic potential6 and survival outcomes7 vs. Gleason 6 tumours, with some authors questioning whether the latter represents cancer at all8,9.
METHODS:
71 men with suspected prostate cancer, or undergoing active surveillance for known prostate cancer were recruited to the study between April and November 2016. Patient demographics are shown in table 1.
Image acquisition
A standard European Society of Urogenital Radiologists (ESUR) compliant multiparametric prostate (mp)-MRI10 was performed in all patients on a 3T scanner (Achieva, Philips Healthcare, NL) supplemented by VERDICT MRI, which employs a series of pulse-gradient spin-echo sequences with various diffusion gradient strengths and timings1.
Diffusion model
VERDICT is a three-compartment model that aims to characterise the fraction of diffusion occurring in the extracellular-extravascular (fEES), intracellular (fIC) and vascular (fvasc) compartments. Cell radius (R) may also be estimated, from which cellularity maps are calculated by dividing fIC by R3.
We fitted the VERDICT model to the VERDICT-MRI data using the AMICO framework11, which uses linearization and convex optimisation for ultrafast fitting. Apparent diffusion coefficient (ADC) was also fitted for comparison. The objective function map (fobj) provides a measure of ‘goodness-of-fit’ allowing voxels with an insufficient fit to be excluded from the analysis. Typical VERDICT maps are shown in figure 1.
Study design
A scan-rescan repeatability study was initially performed in 40 of the 71 patients. 30 of these patients were scanned without an interval between the two scans; hereby defined as ‘immediate’ repeatability and the remaining 10 patients were scanned with a 5-minute interval between, during which time patients walked around the scanner room; hereby defined as ‘interval’ repeatability.
Datasets from the 71-patient cohort were then used to study the relationship between VERDICT metrics and ADC in each Gleason grade. For patients who underwent repeatability examinations, the mean values of their metrics were used.
Image analysis
MR datasets were analyzed using Osirix. A board certified Radiologist manually placed regions of interest (ROI) on all fitted quantitative VERDICT maps, and recorded their mean values (figure 2).
Statistical analysis
To assess the repeatability of VERDICT metrics, intraclass correlation coefficients (ICC) (3,1 with absolute agreement) were calculated. Boxplots were constructed and Kruskal-Wallis with Dunn’s multiple comparison tests performed to determine the distribution of repeatable VERDICT and ADC parameters vs. Gleason grade.
RESULTS:
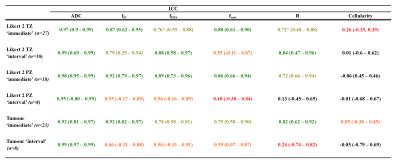
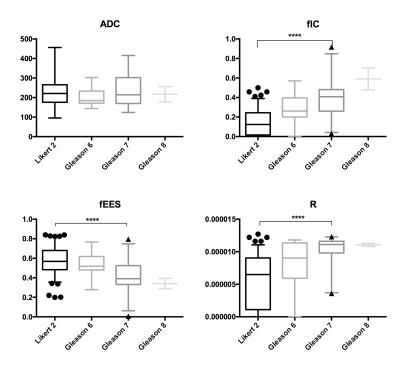
ICCs for ADC and each VERDICT metric are shown in table 2. Boxplots showing the distribution of repeatable VERDICT and ADC parameters in regions that were Likert 2 on mp-MRI and in each Gleason grade (figure 3), with statistically significant results indicated.
DISCUSSION:
We have shown that fIC, fEES fvasc and cell radius VERDICT maps in demonstrate sufficient levels of repeatability for use as clinical tools in most cases. Furthermore, minor improvements in estimation and/or data quality is likely to increase their repeatability further still. The cellularity maps are not sufficiently repeatable at this stage of biomarker development, which arises from instability in the ratio of fIC and R, which can explode for low R – stronger constraints on R may make the parameter more useful.
The repeatable VERDICT maps (other than fvasc) also demonstrate significant clinical potential in that they appear strongly associated with Gleason grade, and appear to identify patients with clinically significant (Gleason 7) tumour, unlike ADC whereby no such capability was found in comparison.
Given these results, there are likely to be multiple potential clinical applications for VERDICT in prostate cancer management, including monitoring patients for progression whilst on active surveillance, appropriately avoiding or triggering prostate biopsies and risk-stratifying patients to make treatment decisions.
CONCLUSION:
We present the first study evaluating the repeatability of VERDICT MRI parameters as prostate cancer imaging biomarkers in a large number of patients, and have confirmed that fIC and fEES, fvasc and cell radius maps are sufficiently repeatable for clinical use. We have also shown that these repeatable biomarkers hold significant clinical potential, in that they appear to distinguish Gleason 7 tumours better than ADC.
Acknowledgements
This work is funded by a grant from Prostate Cancer UK. A grant from the UCLH Biomedical Research Centre supports EJ and SP and EPSRC grant EP/N021967/1 supports EP's work on this topic.References
1 Panagiotaki E, Walker-Samuel S, Siow B, et al. Noninvasive quantification of solid tumor microstructure using VERDICT MRI. Cancer Res 2014; 74: 1902–12.
2 Panagiotaki E, Chan RW, Dikaios N, et al. Microstructural Characterization of Normal and Malignant Human Prostate Tissue With Vascular , Extracellular , and Restricted Diffusion for Cytometry in Tumours Magnetic Resonance Imaging. 2015; 0: 1–10.
3 O’Connor JPB, Aboagye EO, Adams JE, et al. Imaging Biomarker Roadmap for Cancer Studies. Nat Rev Clin Oncol 2016; in press. DOI:10.1038/nrclinonc.2016.162.
4 Johnston E, Pye H, Bonet-Carne E, et al. INNOVATE: A prospective cohort study combining serum and urinary biomarkers with novel diffusion-weighted magnetic resonance imaging for the prediction and characterization of prostate cancer. BMC Cancer 2016; 16: 816.
5 Tefilli M V, Gheiler EL, Tiguert R, et al. Should Gleason score 7 prostate cancer be considered a unique grade category? Urology 1999; 53: 372–7.
6 Ross HM, Kryvenko ON, Cowan JE, Simko JP, Wheeler TM, Epstein JI. Do adenocarcinomas of the prostate with Gleason score (GS) ≤6 have the potential to metastasize to lymph nodes? Am J Surg Pathol 2012; 36: 1346–52.
7 Lau WK, Blute ML, Bostwick DG, Weaver a L, Sebo TJ, Zincke H. Prognostic factors for survival of patients with pathological Gleason score 7 prostate cancer: differences in outcome between primary Gleason grades 3 and 4. J Urol 2001; 166: 1692–7.
8 Ahmed HU, Arya M, Freeman A, Emberton M. Do low-grade and low-volume prostate cancers bear the hallmarks of malignancy? Lancet Oncol 2012; 13: e509-17.
9 Carter HB, Partin AW, Walsh PC, et al. Gleason score 6 adenocarcinoma: Should it be labeled as cancer? J Clin Oncol 2012; 30: 4294–6.
10 Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. Eur Radiol 2012; 22: 746–57.
11 Daducci A, Canales-Rodríguez EJ, Zhang H, Dyrby TB, Alexander DC, Thiran J-P. Accelerated Microstructure Imaging via Convex Optimization (AMICO) from diffusion MRI data. Neuroimage 2015; 105: 32–44.
12 Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159–74.
Figures
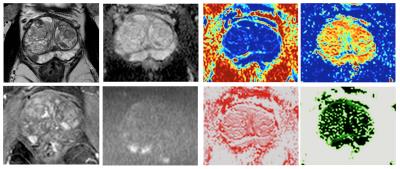
Figure 1.
4 leftmost images: mp-MRI showing Likert 5/5 right PZ tumour at 8 o’clock, with low signal on T2 TSE (top L), low ADC value (top R), early enhancement on dynamic contrast (bottom L) and high signal on b=2000s/mm2 image (bottom R).
4 rightmost images: VERDICT maps of the same tumour: Top L: fIC; High signal tumour representing increased cell volume fraction. Top R: fEES; showing a reduction in the proportion of extravascular, extracellular space. Bottom L: fvasc map showing equivocal appearances. Bottom R: Cellularity map showing high signal in the region of the tumour, reflecting raised cell density.
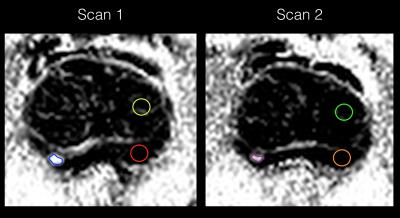
Figure 2. Example of lesion contouring of the tumour in figure 1 as part of a for a repeatability study. Left image: The largest tumour in each zone (TZ/PZ) was contoured (blue in this case). A standard circular 40mm^2 ROI is also placed in normal region (Likert 2) of the TZ (yellow ROI) and PZ (red ROI), as guided by the conventional mp-MRI.
Right image: For the 40 repeatability cases, the ROIs were copied onto the second acquisition and manually adjusted accordingly. (Note images are displayed in black and white for clarity of regions of interest).