1004
Early Cancer Detection Using Paramagnetic Liposome by a Novel Contrast Mechanism with Active-feedback Magnetic Resonance Imaging1DEPARTMENT OF CHEMISTRY AND BIOCHEMISTRY, University Of California, Los Angeles, Los Angeles, CA, United States, 2Department of Chemistry, National Taiwan University, Taiwan
Synopsis
The detection of early tumors requires creating the contrast between healthy and tumor tissues that share a common morphology, making it difficult to distinguish them by relaxation-based MRI technique. Here we have exploited the small magnetic differences between the healthy and tumor tissues to develop a detection technique of early tumors using theranostic nanoparticle paramagnetic (Gd) liposome along with continuous wave (CW) irradiation in the presence of the feedback magnetic field from an
Introduction
Early detection of cancer is critically important to save a significant percentage (about 30%) of those who die of the disease and could significantly increase treatment options. However, conventional magnetic resonance imaging (MRI) techniques based on the measurement of relaxation parameters lack the necessary sensitivity to small differences in tissue pathology characteristic of early tumor growth, because the rapid development of blood vessels associated with the growth of tumor does not appreciably affect the relaxation parameters. Hence, it is necessary to develop new techniques capable of detecting early tumor growth. It is known that early tumor growth changes the local magnetic susceptibility. The goal of this work is to detect small changes in the local magnetic susceptibility associated with the tumor by a non-linear technique that would amplify the small magnetic differences and improve the contrast. One particular source of non-linear evolution is the radiation damping feedback field.1 We are aiming to increase contrast and sensitivity of MRI by manipulating this feedback field along with the targeted nanoparticles to detect very small tumors.Methods
In this work, we developed a novel contrast mechanism using continuous wave irradiation in the presence of the feedback magnetic field from an active-feedback electronic device 2-4 and demonstrated its specific application to detect early tumor growth by using targeted nanoparticle ‘paramagnetic (Gd) liposome’. At first, we incorporated contrast agent Gadolinium (Gd) (III) into the bilayer of the liposome,5 which is a US food and drug administration (FDA) approved nanoparticle capable of targeting cancer cells and acting as a “molecular biomarker”. The nanomaterial was examined and found to be a competent “biomarker” using various characterization methods, such as transmission electron microscopy (TEM), dynamic light spectroscopy (DLS) and inductively coupled mass spectroscopy (ICP-MS). TEM images of the material are shown in Figure 1.
Gd-liposome nanoparticles attach to the tumor site and increase the small magnetic differences at the tumor site by producing a local magnetic field gradient. Our home-built active feedback electronic devices provide feedback pulse sequences to generate avalanching spin amplification, which enhances significantly the MRI contrast of the tumor. The electronic device has been designed to control and optimize the spin amplification process with programmable and controllable feedback phase, gain, and duration for sensitivity enhancement. A continuous wave (CW) was applied to extend the non-linear evolution of the sample by preventing the system from reaching equilibrium.
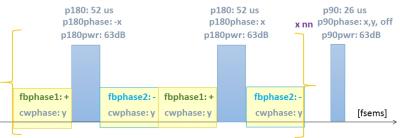
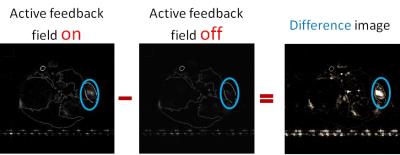
In vivo subcutaneous glioblastoma multiforme (GBM) mouse models were prepared and imaged using conventional and our method. In our method, the images have been acquired twice: once with feedback loop on and then after turning off the feedback loop. The difference of intensity of each point was taken between these two categories of image sequences. The pulse sequence is shown schematically in Figure 2. Computer simulations were carried out to investigate the spin dynamics for this process to support our findings.
Results and Discussion
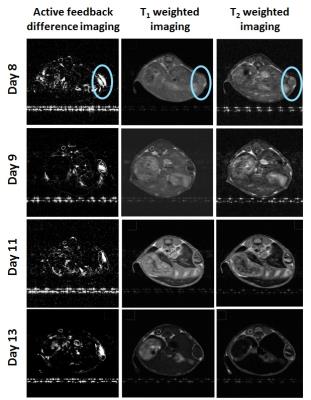
Early stage mice models (8-13 days after injecting tumor cells) shows that conventional T1- and T2-weighted images could not clearly locate the early cancers, whereas this novel contrast mechanism successfully highlighted the early tumors by characterizing “molecular biomarker” Gd-liposome at cancer site with a close correlation to histopathology. Figure 3 and 4 readily show I) enhancement of contrast and higher sensitivity to detect early tumors II) successful targeting to tumor cells. Moreover, our measurements indicate a significant improvement in the contrast-to-noise ratio (CNR) over current conventional imaging methodologies for several mice with early tumors.
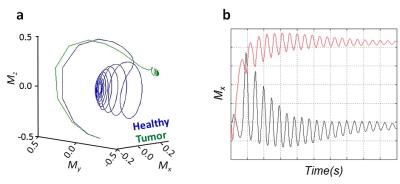
The results from the computer simulations provide further support to this finding. Figure 5 shows the formation of unexpected stable fixed points for healthy and tumor tissues due to the continuous nonlinear evolution of the system which produces a unique sensitivity to small magnetic differences. The resulting dynamics leads to a significant contrast in the spatial distribution of the magnetization, which was spatially encoded and observed by MRI sequences.
Conclusion
We have done both in vivo mouse cancer model experiments and the relevant computer simulations. Our results clearly show that our novel contrast mechanism is very sensitive to small changes in magnetic susceptibility. It could be used as a powerful analytical tool for the robust detection and therapeutics of high-grade malignancy at an early stage. We expect this methodology to produce similar improvements in CNR for human imaging, having the potential to significantly improve early tumor detection in clinical practice. Further, Gd-liposome could be considered as a nano-vehicle for its capability of targeting tumors and carrying drug for future theranostic application, where synergistic detection and therapy of cancer might be possible.Acknowledgements
SR gratefully acknowledges NSF, NSF-EAPSI program, MOST, SIT for the research funding.References
1. Bloembergen N, Pound R.V. Radiation Damping in Magnetic Resonance Experiments. Phys. Rev.1954; 95 (1): 8-12.
2. Lin Y, Lisitza N, Ahn S, et al. Resurrection of Crushed Magnetization and Chaotic Dynamics in Solution NMR spectroscopy. Science 2000; 290 (5489):118-121.
3. Weiss P, Magnetic Whispers: Chemistry and Medicine Finally Tune into Controversial Molecular Chatter. Science News 2001; 159 (3):42-44.
4. Li Z, Hsu C, Dimitrov N, et al. Sensitive Imaging of Magnetic Nanoparticles for Cancer Detection by Active Feedback MR. Magnetic Resonance in Medicine 2015; 74 (1):33-41.
5. Mulder WJM, Strijkers GJ, Griffioen AW, et al. A liposomal system for contrast-enhanced magnetic resonance imaging of molecular targets. Bioconjug Chem. 2004;15 (4):799-806.
Figures