1003
Visualizing local mechanical properties of agar phantoms and meningioma patients using magnetic resonance rheology1Helmholtz-Institut für Strahlen- und Kernphysik, University of Bonn, Bonn, Germany
Synopsis
Magnetic resonance rheology is a novel method to create an imaging contrast based on the mechanical properties of brain tissue. It is based on a short fall of the head that creates a broadband excitation of the tissue. The resulting deflections of the tissue elements are depicted using motion sensitive phase imaging. This contribution presents measurements on agar phantoms as well as four meningioma patients to show the feasibility of the method to depict local alterations of the mechanical properties of the investigated material.
Introduction
Magnetic Resonance Rheology (MRR)1 is a novel method to visualize the mechanical properties of brain tissue. The main difference to the more common magnetic resonance elastography is its means of excitation: MRR uses a short fall of the head to create a global excitation of the brain tissue. The introduced deflection of the tissue elements are measured using motion sensitive MRI phase imaging. We present measurements on two agar-based phantoms and a study on four patients diagnosed with meningioma to show the feasibility of this method to visualize locally different mechanical properties of the material based on the phase contrast.Methods
MRR uses a custom build lifting device (Fig. 1) adapted to a standard head coil of a Siemens Avanto 1.5 T scanner. The patient’s head respectively the phantom is positioned on a moveable shell that is lifted pneumatically to a height of approximately 1 mm. It is then dropped synchronized to an SE-EPI sequence equipped with two identical trapezoidal motion encoding gradients2 (MEG) in the direction of the falling motion on either side of the inversion pulse. The falling motion excites a wide band of frequencies in the investigated material. The resulting deflection is sampled by shifting the start of the falling motion (parameter $$$\tau$$$) (Fig. 2). The first presented study was on two cylindrical phantoms each filled with two different layers of agar based hydrogel3. One phantom consisted of two layers with different agar concentration and thus different Young’s moduli, the second phantom consisted of two layers of equal Young’s moduli but different density, which was achieved by adding tungsten carbide powder to the hydrogel. The second study was performed on four meningioma patients prior to surgery. In both studies, phase images of one transversal slice with induced motion were acquired at different tau values to examine the phase progression per voxel with respect to tau. The imaging parameters were TR = 3000 ms resp. 3500 ms (phantoms resp. meningioma patients), TE = 80 ms, matrix size: 112 x 128, voxel size between 1.4 x 1.4 x 5 mm and 2 x 2 x 5 mm. The strength of the MEGs was G = 34.8 mT/m, the length of one MEG was delta = 5 ms and the distance between both MEGs was Delta = 45 ms. All measurements were accompanied by a measurement with the same parameters but without induced motion of the head that served as a baseline measurement. The images were post-processed by subtraction of the baseline, normalization to a reference point and numerical phase unwrapping. As a measure of strain instead of deflection, the gradient of the phase in the direction of the falling motion was calculated after smoothing with a gaussian filter ($$$\sigma$$$ = 0.6).Results
The phase images of the phantoms clearly show the different oscillatory behavior of the two layers. This distinction is even easier in the calculated strain images (cf. Fig 3). For the meningioma patients, the tumor regions of all four patients show up in the motion images with a distinct signature of less deflection. In the strain images, the tumor regions show reduced strain values (cf. Fig. 4)Discussion
The phantom measurements showed that the signal measured in MRR is dependent both on the elastic properties and the density of the material. In vivo, the typical phase signal of an MRR measurement is a global oscillation of the brains hemispheres with a higher deflection of tissue elements in the center of the hemisphere than near to the meninges, as was shown in previous studies1. As expected, the different mechanical properties of the meningioma tissue lead to a different $$$\tau$$$-dependence of the measured phase in those regions. All meningiomas were classified by the surgeons as significantly stiffer than healthy tissue, which explains the lower strain values found in those regions.Conclusion
The presented results show the feasibility of MRR to visualize local alterations of the mechanical properties of brain tissue. To investigate the full potential of this method, however, the number of patients in this study was too small. Further research will address this, and will attempt to quantify the results of MRR measurements with the aim to infer the mechanical properties of the investigated brain tissue.Acknowledgements
The authors would like to thank Prof. Elke Hattingen and Dr. Bogdan Pintea for their valuable help with the study on meningioma patients and Dr. Jürgen Finsterbusch for the development of the MRI sequence.References
1) Kofahl A-L, Theilenberg S., et al. Combining Rheology and MRI: Imaging Healthy and Tumorous Brains Based on Mechanical Properties. Magn Reson Med 2016; doi:10.1002/mrm.26477.
2) Stejskal EO, Tanner JE. Spin diffusion measurements: spin echoes in the presence of a time-dependent field gradient. J Chem Phys 1965;42:288-292.
3) Konofagou EE, Hynynen K. Localized harmonic motion imaging: theory, simulations and experiments. Ultrasound Med Biol 2003;29:1405–1413.
Figures
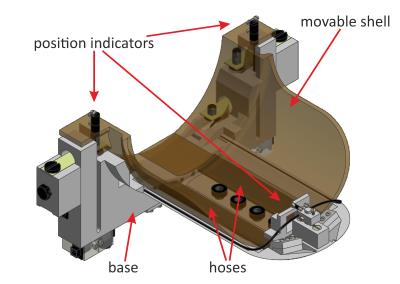
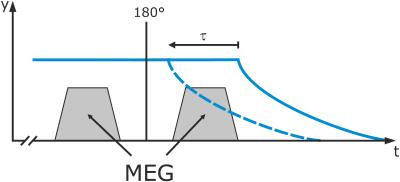
Figure 2: Motion sampling: MRR uses two trapezoidal gradients in the direction of the falling motion on either side of the refocusing pulse of a 2D spin-echo echo-planar imaging sequence. Prior to the measurement, the movable shell is in the upper position. It is then dropped synchronized to the imaging sequence.
By shifting the start of the falling motion relative to the sequence, the temporal behavior of the sample can be investigated. This is described by the parameter $$$\tau$$$ that is defined as zero in the case that all motion encoding stops before the start of the falling motion.
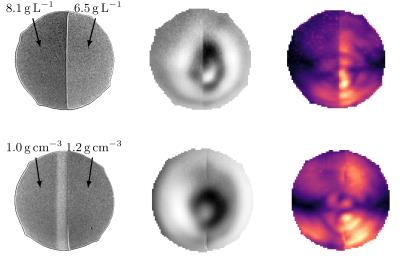
Figure 3: Exemplary results of the phantom measurements: From left to right: High resolution TSE scan, exemplary motion image, phase strain image. here, lighter colors represent higher strain values.
Upper line: Two layered phantom with different agar concentrations. The phase image shows larger deflection in the softer layer. Correspondingly, the phase strain values in this layer are higher.
Lower line: Two layered phantom with different densities. The phase image does not show a clear distinction between both layers. The phase strain shows different values though, albeit the difference is smaller than the one observed in the other phantom.
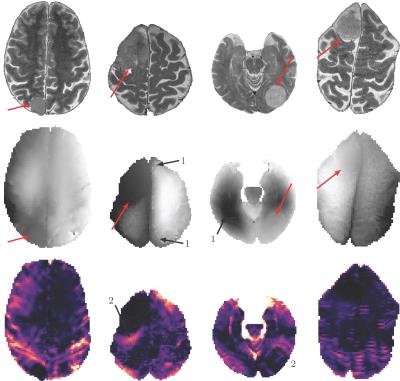
Figure 4: Exemplary images of the four Meningioma patients:
The upper line shows TSE images, the middle line phase images and the lower line the calculated total absolute phase strain. Here, lighter colors represent higher strain values. Red arrows indicate the tumor region and (1) indicates unwrapping artifacts in the phase images.