0999
Magneto-Caloric Materials as Tunable and Switchable Labels for MRI1Applied Physics and Instrumentation Group, HHMI-Janelia Research Campus, Ashburn, VA, United States, 2Laboratory of Functional and Molecular Imaging, NIH/NINDS, Bethesda, MD, United States, 3NIH Mouse Imaging Facility, NIH/NINDS, Bethesda, MD, United States, 4Code 5611, Optical Sciences Division, Naval Research Laboratory, Washington, DC, United States, 5Quantum Design, Inc., San Diego, CA, United States
Synopsis
We present the case for the use of magneto-caloric materials as tunable and switchable labels for MRI. Sharp magnetic phase transitions these materials have at typical physiological temperatures and in the presence of the large DC magnetic field values associated with MRI machines make them uniquely suitable for the development of novel MRI contrast agents. We present physical and MRI measurements of a prototypical magneto-caloric material Iron-Rhodium (FeRh) that clearly demonstrate the MR image contrast changes due to the temperature tunable magnetic state of the material in the MRI compatible magnetic field range and physiologically relevant temperature range.
Introduction
Development of novel contrast mechanisms and labeling agents for MRI is critical for further advancements in non-invasive cell imaging, tracking, and readout of physiological conditions in-vivo1-4. Recent advances in magneto-caloric materials (developed for magnetic refrigeration5 and data storage applications6) may provide an opportunity to apply these materials as tunable and switchable contrast agents for MRI. More specifically, the extremely sharp first-order magnetic phase transitions these materials have at typical physiological temperatures and in the presence of the large DC magnetic field values associated with MRI machines provide an ideal match to the requirements for the design of novel MRI labels. Furthermore, a wide range of magneto-caloric materials are available that can be engineered and fine-tuned to optimize their response under MRI-appropriate conditions. We present physical and MRI measurements of a proto-typical magneto-caloric material, Iron-Rhodium (FeRh)7-8, to develop the case for the use of such materials for MRI.Methods
Iron-Rhodium granules (Fe 49%, Rh 51% atomic composition, 99.9% purity) were prepared by mixing in an arc melting furnace (American Elements Corp.), followed by high-temperature annealing in Argon gas furnace at 1,000°C for two weeks, and subsequently quenched in ice-water. The samples were then wire cut into mm-scale discs for magnetic measurements in a 9T vibrating sample magnetometer (Quantum Design, Inc.). In order to demonstrate the feasibility of a magneto-caloric material as a tunable and switchable contrast agent in typical MRI settings, a 4.7T MRI (Bruker Biospin, Inc.) was used because this was the available polarizing DC magnetic field that is closest to the value where the sharp first order transition happens near the physiological temperature of 37°C (310K). For the MRI characterization, the sample was embedded in agarose next to a MRI-compatible optical fiber-based thermometer (FISO Technologies, Inc.). The sample tube was wrapped in water tubing connected to a temperature controlled water circulating bath in order to sweep and control the temperature of the sample and its environment around physiologically relevant conditions (15-55°C).Results
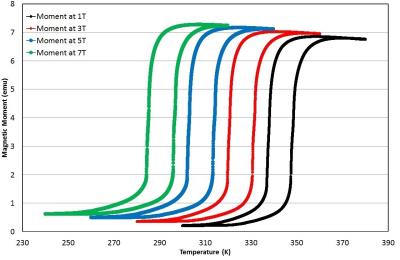
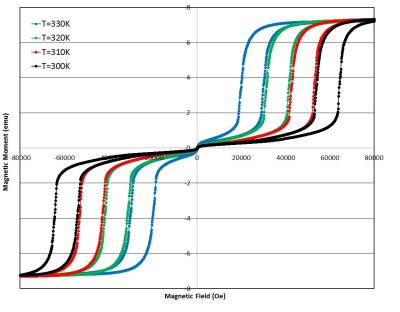
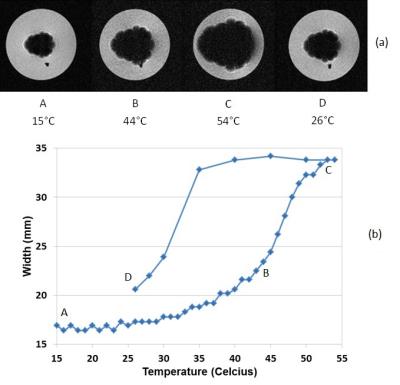
Figure 1 shows the measurement of the magnetic moment of a typical FeRh disk sample as a function of temperature in different bias DC magnetic fields. The sample exhibits a sharp transition from an antiferromagnetic to a ferromagnetic state over a very narrow range of physiologically relevant temperatures. More specifically, the sample has a sharp transition around body temperature (37°C = 310K) in the DC bias field of around 5 Tesla. The sample magnetization can also be tuned with the magnetic field, as Figure 2 shows. In this measurement, the moment is measured as a function of the magnetic field at a constant temperature. The sharp transition is again present at large DC magnetic field values and around physiologically relevant temperatures. Figure 3(a) shows representative gradient-echo images of the effect of the mm-scale disk of FeRh on the surrounding agarose as the temperature is swept from the antiferromagnetic phase below the transition temperature to the ferromagnetic phase above the transition temperature of the FeRh sample and then cooled. The size of the region with signal dropout due to high magnetic field gradients approximately doubles in each dimension, a factor of 8 in volume. Loss of signal in MRI due to the changing magnetic state of the material closely follows the magnetic properties shown in Figure 1 with a temperature tunable magnetic state of the sample. This is plotted in Figure 3(b) which shows the MRI signal loss region size (in a linear dimension) vs. temperature. (Image parameters: TR/TE = 100/2.2 ms, FA = 25 degrees, nominal resolution = 0.46 x 0.46 x 1mm, FOV = 60.0 x 60.0 mm).Discussion and Conclusion
The clearly demonstrated phase shift with concomitant magnetic field change is seen in the increase of the MRI signal void in Figure 3. There was a larger hysteresis and lower apparent moment increase in the MRI data than in the magnetometer data which is likely related to mechanical stress when cutting the material to smaller size. This hysteresis effect could be viewed as a benefit, in that once the particle is turned ‘on’ it will remain on until removed from the field. The remaining challenges and opportunities using magneto-caloric materials and the research path being pursued towards functionalizing these materials as labels in MRI will be discussed. In particular, progress in preparing them in microparticle form, tuning their physical properties through alloying, and designing the temperature and magnetic field instrumentation for controlled switching of these materials for in-vivo applications will be presented.Acknowledgements
This research was supported in part by the Howard Hughes Medical Institute and the NINDS Intramural Research Program of National Institutes of Health.References
1. E. M. Shapiro, S. Skrtic, K. Sharer, J. M. Hill, C. E. Dunbar, and A. P. Koretsky, MRI detection of single particles for cellular imaging, Proc. Natl. Acad. Sci. U S A. 101(30): 10901–10906 (2004).
2. G. Zabow, S. Dodd, J. Moreland, and A. P. Koretsky, Micro-engineered local field control for high-sensitivity multispectral MRI, Nature 453, 1058 (2008).
3. E. T. Ahrens and J. W. M. Bulte, Tracking immune cells in vivo using magnetic resonance imaging, Nature Reviews Immunology, 13, 755 (2013).
4. G. Zabow, S. Dodd, and A. P. Koretsky, Shape-changing magnetic assemblies as high-sensitivity NMR-readable nanoprobes, Nature 520, 73 (2015).
5. J. Liu, T. Gottschall, K. P. Skokov, J. D. Moore and O. Gutfleisch, Giant magnetocaloric effect driven by structural transitions, Nature Materials 11, 620 (2012).
6. J. U. Thiele, S. Maat, and E. E. Fullerton, FeRh/FePt exchange spring films for thermally assisted magnetic recording media, Appl. Phys. Lett. 82, 2859 (2003).
7. J. S. Kouvel and C. C. Hartelius, Anomalous Magnetic Moments and Transformations in the Ordered Alloy FeRh, J. Appl. Phys. 33, 1343 (1962).
8. J. S. Kouvel, Unusual Nature of the Abrupt Magnetic Transition in FeRh and Its Pseudobinary Variants, J. Appl. Phys. 37, 1257 (1966).
Figures