0988
Multi-parametric MRI Radiomics for Pre-treatment Prediction of the Progression-Free Survival in Advanced Nasopharyngeal Carcinoma1Guangdong General hospital, Guangzhou, People's Republic of China
Synopsis
To our knowledge, this is the first MRI-based radiomics study to predicting survival of tumor. The results of our study show that multiparametric MRI-based radiomics nomogram significantly improves the 7th edition of AJCC TNM staging system and clinical data in predicting individualized progression-free survival (PFS) in advanced NPC (stage III-IVb). In fact, the radiomics nomogram built in our study could integrate all prognostic biomarkers/signatures that have been published in this area to improve its predictive performance. Besides, for the first time, our radiomics heatmaps showed positive associations between radiomics signature features with overall stage, T-stage and negative associations between radiomics signature features with N-stage. Our radiomics study provides some different insights into the mechanism of hematogenous and lymphatic metastasis of NPC.
Introduction
The main causes for treatment failure of nasopharyngeal carcinoma (NPC) is locoregional recurrences and distant metastasis. Pre-treatment prediction of recurrence and distant metastasis in patients with NPC is crucial for prognosis and treatment decision making. Knowledge of poor survival before treatment can provide valuable information for deciding the need for aggressive treatment, such as increasing cycles or using gemcitabine plus cisplatin instead of fluorouracil plus cisplatin as the standard first-line treatment option. This study aimed to identify MRI-based radiomics for the pre-treatment prediction of progression-free survival (PFS) in patients with advanced nasopharyngeal carcinoma (NPC) (stage III-IVb) .Methods
Our Institutional Review Board approved this retrospective study and waived the need to obtain informed patient consent. A total of 118 patients (training cohort: n = 88; validation cohort: n = 30) with advanced NPC received pre-treatment 1.5 T MRI scans from January 2007 to August 2013 in our institution. A total of 970 radiomics features per patient were extracted from T2-weighted (T2w) and contrast-enhanced T1-weighted (CET1w) MRI of NPC. LASSO regression model was applied for data dimension reduction, feature selection and radiomics signature building. We firstly constructed a clinical nomogram to integrate clinical data including the TNM staging system, age, gender, hemoglobin, and platelet counts and then presented a radiomics nomogram to integrate radiomics signature or rad-score with clinical data. Discrimination (C-index) and calibration were used to evaluate radiomics performance. In addition, we investigated the association of radiomics features with clinical data using heat map. Statistical analysis were performed with R software using the packages: “glmnet”, “survival”, “rms” “Hmisc”,“ResourceSelection”, “gplots” and “pheatmap”. All statistical tests were two-sided, and p-values of < 0.05 were considered significant.Results
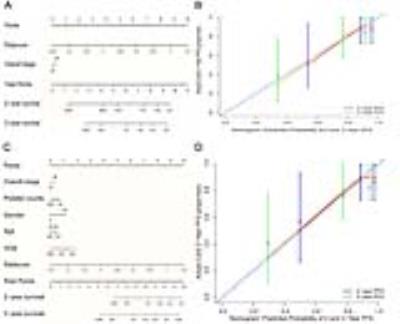
Eight MRI-based radiomics signature that significantly associated with PFS was identified from 970 features. The prognostic value of radiomics signature derived from combined CE T1w and T2w images performed better than that from CET 1w or T2w images alone. The TNM staging system yielded a C-index of 0.514 (95%CI: 0.432 to 0.596).The radiomics nomogram integrated radiomics signature from combined CET1w and T2w images with the TNM staging system showed a significant improvement of the TNM staging system in predicting PFS in the training cohort (C-index, 0.761 vs 0.514; p < 2.68 × 10-9) (Figure 1A). The clinical nomogram yielded a C-index of 0.649 (95%CI: 0.552 to 0.746). The radiomics nomogram integrated radiomics signature with clinical data outperformed the clinical nomogram (C-index, 0.776 vs 0.649; p < 1.60 × 10-7) (Figure 1C). The calibration curves showed the good agreements between nomogram-predicted and actual survival (Figure 1B and 1D). These results were further confirmed in the validation cohort. Radiomic heatmaps suggested associations between radiomics features with tumor stages (Figure 2).Discussion
The present study developed and validated multi-parametric MRI- based radiomics as a convenient approach to predict individual PFS pre-treatment in patients with advanced NPC (stage III-IVb). The radiomic signature presented complementary value to the 7th AJCC TNM staging system and clinical data for individualized PFS estimation. Such quantitative radiomics prognostic models of NPC may potentially be useful for precision medicine and affect patient treatment strategy.Conclusion
Multiparametric MRI-based radiomics nomogram shows more accurate than the traditional TNM staging system and clinical nomogram in predicting individualized PFS in advanced NPC, which epitomizes the pursuit of precision medicine and can be used to improve patient treatment strategy.Acknowledgements
This study was supported by the National Scientific Foundation of China (81571664) and the Science and Technology Planning Project of Guangdong Province (2014A020212244, 2016A020216020).References
Aerts, H. J. et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 5, 4006 (2014).Figures