0987
A preclinical MRI study investigating the impact of the local microenvironment on the progression of diffuse intrinsic pontine glioma in patient-derived xenografts1The Institute of Cancer Research, London, United Kingdom
Synopsis
Diffuse intrinsic pontine glioma (DIPG) is a devastating childhood brain tumour with very poor outcome. The local brain microenvironment appears to play an important role in the tumourigenesis of DIPG, and is currently underinvestigated. Infratentorial and hemispheric tumour growth patterns of orthotopic DIPG xenografts were assessed longitudinally by anatomical MRI, and confirmed by immunohistochemical analysis. ADC, T1 and T2 were higher in infratentorial tumours than hemispheric tumours, corresponding to a higher degree of tumour-associated oedema observed histologically.
Introduction
Diffuse intrinsic pontine glioma (DIPG) is an incurable paediatric brain tumour with very poor survival (90% mortality within 18 months)1. Tumours arise in the ventral pons, part of the brainstem, and widely infiltrate adjacent structures2.
To date, little is known about the role of the local environment in the malignancy of DIPG. Investigating whether the local brain microenvironment affects the growth of DIPG cells may help to evaluate brain location-dependent influences on tumour promotion and progression.
In this study, multiparametric MRI was performed on mice with DIPG cells orthotopically implanted either in the pons or the cerebral hemisphere, to identify and compare tumour growth and radiological phenotype, and assess the impact of the local microenvironment on the progression of DIPG.
Methods
Primary patient-derived SU-DIPG-VI cells were expanded on laminin under stem cell culture conditions. 2.5x105 cells were intracranially injected in 6-week old nude mice, either in the pons or the right frontal lobe (hemispheric).
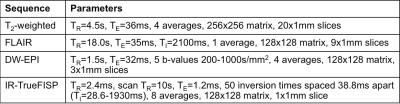
MRI was carried out on a 7T Bruker horizontal bore microimaging system using a 3cm birdcage coil over an axial 2.5cmx2.5cm field-of-view. Anatomical T2-weighted MRI and FLAIR imaging were applied weekly from day 20 post-injection to localise the tumour and monitor disease progression. At study end, diffusion-weighted echo-planar imaging (DW-EPI) and inversion recovery (IR)-trueFISP MRI were performed, in addition to anatomical measurements. Sequence parameters are shown in Table 1.
Parametric apparent diffusion coefficient (ADC), T1, and T2 maps were generated by fitting the data on a pixel-by-pixel basis with a Bayesian maximum a posteriori algorithm using in-house software3. Tumour regions-of-interest (ROIs) were analysed to quantify median ADC, T1, and T2 values.
Serial 4µm sections were cut from formalin-fixed paraffin-embedded brain tissue, and stained with haematoxylin and eosin (H&E), or immunohistochemically processed for the detection of human nuclear antigen (HNA) to identify tumour cells.
Results
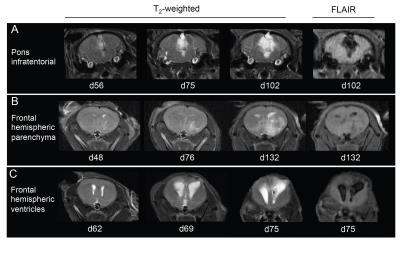
SU-DIPG-VI cells injected into the pons formed tumours within infratentorial regions of the brain (pons, cerebellum, fourth ventricle) (Figure 1A), and injections into the cerebral hemisphere resulted either in tumours within the hemispheric parenchyma (Figure 1B), or expansion of the ventricular system, suggestive of tumour involvement (Figure 1C).
Infratentorial tumours appeared brighter on T2-weighted images than hemispheric tumours (Figure 1A, 1B). Signal attenuation on FLAIR images at study end was prominent in infratentorial tumours, but only evident in small areas within hemispheric tumours.
FLAIR imaging of mice with putative ventricular tumours (Figure 1C) showed fully and partially attenuated regions within the lateral ventricles and the hemispheric parenchyma adjacent to the lateral ventricles.
Median survival of mice injected with SU-DIPG-VI cells in the cerebral hemisphere was longer (118 days) than those with tumours propagated in the pons (100 days).
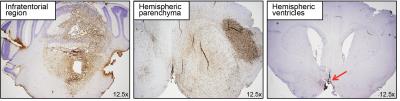
HNA immunohistochemistry showed diffusely distributed SU-DIPG-VI tumour cell nuclei within both infratentorial regions following pontine cell injections, and the hemispheric parenchyma following hemispheric injections (Figure 2). Tumour cells were identified in the third ventricles of mice with expanded lateral ventricles following hemispheric injection, confirming tumour growth within the ventricular system.
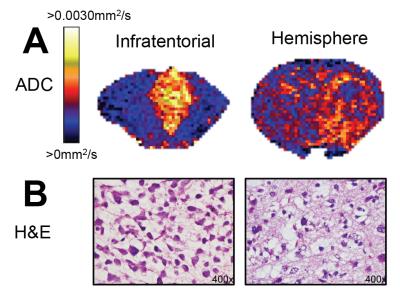
ADC maps showed tumour heterogeneity within T2w-hyperintense lesions in both infratentorial and hemispheric tumours (Figure 3A). Regions of high ADC corresponded to FLAIR attenuation in both locations, and were more prominent in infratentorial tumours than hemispheric tumours. H&E showed oedema in all tumours, which was more substantial within infratentorial tumours than hemispheric tumours (Figure 3B).
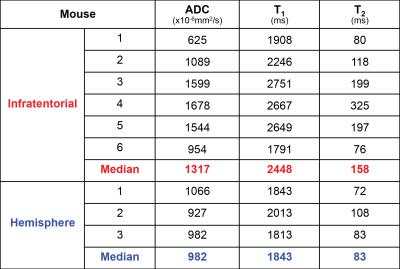
Tumour ADC, T1, and T2 were quantified, and all parameters were higher in infratentorial tumours than hemispheric tumours (Table 2).
Discussion
Prominent tumour progression in the lateral ventricles and throughout infratentorial structures underscores the ability of DIPG cells to disseminate to other regions of the brain. DIPG tumour growth in patients has been identified in the cerebellum, thalamus, and frontal lobe4.
Survival was shorter for mice with infratentorial tumours than those with hemispheric tumours, possibly due to more restricted infratentorial volume, and the role of the surrounding structures in vital processes, such as respiratory and cardiac function.
SU-DIPG-VI tumour cell nuclei were found in brain structures abnormal on anatomical MR images, corroborating its high accuracy to track tumour growth patterns in this disease.
Radiologic intratumoural heterogeneity is commonly found in DIPG patients, and includes peritumoural oedematous components5. The high tumour ADC values observed in SU-DIPG-VI tumours corresponds to the presence of tumour-related oedema, and the resulting low cellularity. Infratentorial tumours caused more oedema than hemispheric tumours, suggesting that brain location may influence fluid accumulation in tumours.
Conclusion
This study demonstrates that different local brain microenvironments influence tumour phenotype of DIPG in preclinical models.Acknowledgements
We acknowledge the Cancer Research UK (CR-UK) support to the Cancer Imaging Centre at The Institute of Cancer Research (ICR) and Royal Marsden Hospital (RMH), in association with MRC and the Department of Health C1060/A16464, and NHS funding to the NIHR Biomedical Research Centre.References
1. Bailey S, Howman A, Wheatley K, et al. Diffuse intrinsic pontine glioma treated with prolonged temozolomide and radiotherapy--results of a United Kingdom phase II trial (CNS 2007 04). Eur J Cancer. 2013;49(18):3856-3862.
2. Misuraca KL, Cordero FJ, Becher OJ. Pre-clinical models of diffuse intrinsic pontine glioma. Front Oncol. 2015;5:172.
3. Walker-Samuel S, Orton M, Boult JK, Robinson SP. Improving apparent diffusion coefficient estimates and elucidating tumor heterogeneity using Bayesian adaptive smoothing. Magn Reson Med. 2011;65(2):438-447.
4. Caretti V, Bugiani M, Freret M, et al. Subventricular spread of diffuse intrinsic pontine glioma. Acta Neuropathol. 2014;128(4):605-607.
5. Clerk-Lamalice O, Reddick WE, Li X, et al. MRI Evaluation of Non-Necrotic T2-Hyperintense Foci in Pediatric Diffuse Intrinsic Pontine Glioma. AJNR Am J Neuroradiol. 2016.
Figures