0986
Tumor Interstitial Fluid Pressure and Hydraulic Conductivity Estimates by DCE-MRI in a Rat Model of Cerebral Tumor1Neurology, Henry Ford Hospital, Detroit, MI, United States, 2Physics, Oakland University, Rochester Hills, MI, United States, 3Neurosurgery, Henry Ford Hospital, Detroit, MI, United States, 4Radiation Oncology, Henry Ford Hospital, Detroit, MI, United States
Synopsis
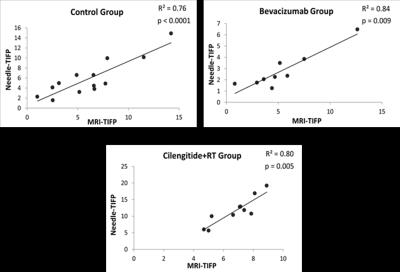
An elevated tumor interstitial fluid pressure (TIFP) is a critical element for assessing therapeutic response. This study demonstrates the use of DCE-MRI to estimate TIFP, and validates that estimate by an invasive method in a rat glioblastoma model, with and without treatment interventions. Significant positive correlations between MRI-derived TIFP estimates and invasive measures of TIFP were found in all groups (e.g., for untreated group, R2=0.76, p<0.0001). These findings validate an MRI-estimated TIFP as a noninvasive measure of TIFP in embedded cerebral tumors, and suggest that it may be a useful tool in assessing tumor response to therapy.
Purpose:
To use dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) to estimate tumor Interstitial fluid pressure (TIFP) and hydraulic conductivity in a rat model of embedded cerebral tumor; to validate that estimate with a direct invasive measure of TIFP. TIFP, elevated in most solid tumors, limits central perfusion in a tumor and thus limits the efficacy of common therapies. An elevated TIFP is considered a mark of aggressiveness, and a decreased TIFP is a predictor of response to therapy1. TIFP has been measured by such invasive methods as the wick-in-needle (WIN) technique, but this direct measure is impractical in many tumor types, and in repeated studies.Methods:
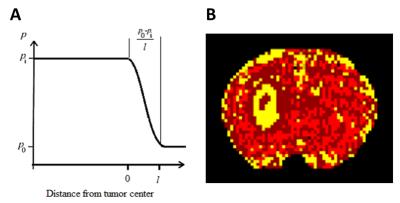
A model for estimating TIFP was developed, based on the mean flux of the contrast agent Magnevist at the tumor periphery; this flux is driven by the IFP gradient at the tumor surface (see Fig.1), and by such characteristics of the interstitium as porosity and interstitial hydraulic conductivity. Twenty-two athymic rats were inoculated intracerebrally with U251 glioblastoma tumor cells. DCE-MRI was conducted in control, bevacizumab-treated, and cilengitide+RT-treated rats, followed by a needle-based WIN measurement of TIFP for each animal. By using model selection to define the edge of the tumor, and the application of Patlak and Logan plots to DCE-MRI data, extracellular volume fraction, VD (i.e., porosity) in the tumor2, and velocity of exudate fluid at the tumor surface were derived3. Hydraulic conductivity was estimated for each animal by using the Kozeny–Carman equation4, which depends mainly on VD, and TIFP was estimated via Darcy’s law4, which relates the IFP gradient and the velocity of exudate fluid at the tumor surface through hydraulic conductivity. TIFP estimates were compared to WIN measures performed immediately after the DCE-MRI study.Results:
The mean of the estimated hydraulic conductivity (MRI-K) in the control group was (2.3 ± 3.1) x 10-5 [mm2/mmHg-s], which is in agreement with other reports. See Fig. 2, showing significant positive correlations between WIN-TIFP and MRI-TIFP in all groups. In the control group, MRI-TIFP was a strong predictor of WIN-TIFP (R2 = 0.76, p<0.0001). Similarly, in the treated Bevacizumab and Cilengitide+RT groups, MRI-TIFP remarkably predicted WIN-TIFP ( R2 = 0.84, p= 0.009), and (R2 = 0.80, p = 0.005), respectively.Discussion:
These results demonstrate that, if hydraulic conductivity can be estimated from the measured porosity of the tissue, then TIFP can be noninvasively estimated. Neglecting the individual tumor’s differences in hydraulic conductivity in determining TIFP may lead to substantial systematic errors in estimation of TIFP. A poor correlation between the MRI-TIFP and WIN-TIFP (R2=0.13, p= 0.23) occurred when a fixed value of hydraulic conductivity was applied in our model instead of the MRI-K for the control group. A multivariate analysis of WIN-TIFP and MRI tumor vascular parameters confirmed these results, showing that VD in the tumor (i.e., tumor porosity), the main factor in estimating MRI-K, was found to be the most tumor parameter to describe TIFP with p= 0.0002.Conclusion
These findings demonstrate that TIFP can be estimated noninvasively using DCE-MRI in embedded cerebral tumors. Since the methods are noninvasive, they might be a useful tool in both pre-clinical and clinical studies for assessing tumor response to therapy.Acknowledgements
The authors thank Ms. Jun Xu for her superb technical assistance.References
1) Lunt SJ, et al. Interstitial fluid pressure in tumors: therapeutic barrier and biomarker of angiogenesis. Future oncology (London, England). 2008;4(6):793-802
2) Aryal MP, et al. Dynamic contrast enhanced MRI parameters and tumor cellularity in a rat model of cerebral glioma at 7 T. Magnetic resonance in medicine. 2014;71(6):2206-14.
3) Ewing JR, et al. Peritumoral tissue compression is predictive of exudate flux in a rat model of cerebral tumor: an MRI study in an embedded tumor. NMR in biomedicine. 2015;28(11):1557-69.
4) Truskey GA, et al.. Transport Phenomena in Biological Systems: Pearson Prentice Hall; 2010.
Figures