0976
Automated identification of hypoxia-related regional variations in tumour microenvironment using dynamic contrast-enhanced MRI and oxygen-enhanced MRI1Division of Informatics, Imaging and Data Sciences, The University of Manchester, Manchester, United Kingdom, 2CRUK & EPSRC Cancer Imaging Centre in Cambridge and Manchester, Cambridge and Manchester, United Kingdom, 3Division of Molecular and Clinical Cancer Studies, The University of Manchester, Manchester, United Kingdom, 4Department of Radiology, The Christie NHS Foundation Trust, Manchester, United Kingdom, 5Division of Pharmacy & Optometry, The University of Manchester, Manchester, United Kingdom, 6Bioxydyn Ltd., Manchester, United Kingdom
Synopsis
Hypoxia is an important prognostic indicator in most solid tumours. We present here automated, data-driven methods, using principal component analysis (PCA) and Gaussian mixture modelling (GMM), that consistently locate functionally distinct sub-regions in preclinical tumours, some of which are postulated to be relevant to hypoxia. Methods are based on dynamic contrast-enhanced (DCE)-MRI (reflecting perfusion) and oxygen-enhanced (OE)-MRI (reflecting oxygen delivery). We demonstrate the utility and stability of our methods through a combination of evaluation metrics, which may be incorporated in similar studies elsewhere.
Introduction
Tumours are biologically heterogeneous.[1,2] Previous work has shown that biomarkers from combined DCE-MRI and OE-MRI can identify hypoxic regions within tumours.[3,4] This is of interest since hypoxia is an important negative prognostic factor in most solid tumours. The current work builds on this by exploring patterns in combined DCE-MRI and OE-MRI data, using automated, objective, and data-driven methods and with a thorough assessment of methodological optimisation and stability.Methods
1. Imaging
9 U87 (glioblastoma) and 7 Calu6 (non small cell lung carcinoma) xenografts were grown in the lower midline of the back of nude mice. Each mouse underwent OE-MRI followed by DCE-MRI on a 7 T Bruker system, while anaesthetised with 2% isoflurane carried initially in medical air (21% oxygen). OE-MRI consisted of a variable flip angle (VFA) spoiled gradient echo (SPGR) acquisition to calculate native tissue $$$T_1$$$ ($$$T_{10}$$$), followed by 42 dynamic $$$T_1$$$-weighted SPGR acquisitions at a temporal resolution of 28.8 s, during which the gas supply, administered via a nose cone, was switched from air to pure oxygen at acquisition 19. DCE-MRI consisted of a VFA sequence again to calculate tissue $$$T_{10}$$$ values, followed by 96 dynamic $$$T_1$$$-weighted SPGR acquisitions at a temporal resolution of 5.78 s, during which Gd-DOTA was injected into a tail vein at acquisition 25.
2. Processing
OE and DCE signals were converted to $$$\Delta R_1$$$ (change from native $$$R_1$$$ where $$$R_1 = \frac{1}{T_1}$$$) and corrected for baseline drift. OE $$$\Delta R_1$$$ values were then scaled so the standard deviation across all voxels’ time series matched that of the DCE data. Features were then extracted using PCA on concatenated DCE-OE $$$\Delta R_1$$$ time course data across all 16 animals’ tumour voxels.
3. Clustering
Prominent clusters in the PCA-derived feature set were located using GMM, with the number of clusters ($$$N_C$$$) varying from 2-25. For each $$$N_C$$$ value, mean within-cluster DCE and OE curves were calculated, and cluster assignments transferred into image space to create tumour region maps.
4. Evaluation
For each $$$N_C$$$, the Akaike information criterion ($$$AIC$$$) was calculated. Technique efficacy was investigated by comparing measures of regional contiguity within region maps to null distributions of contiguity generated using random resampling, and calculating contiguity z-scores. Methodological stability was assessed using bootstrap resampling of the feature distribution and quantifying the stability of the location of cluster centres across bootstrap realisations using silhouettes[5].
Results
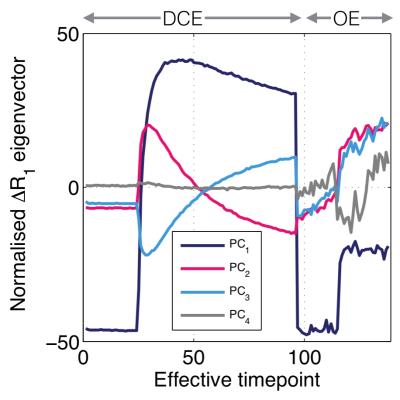
PCA identifies distinct kinetics of enhancement from the group of tumours, as shown in Fig. 1, with the first four principal components explaining over $$$99\%$$$ of the variance within the data set. This provided us with justification for choosing a four-dimensional feature set.
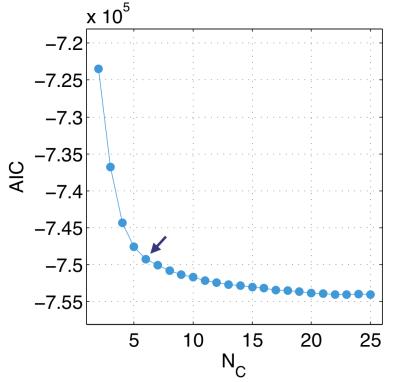
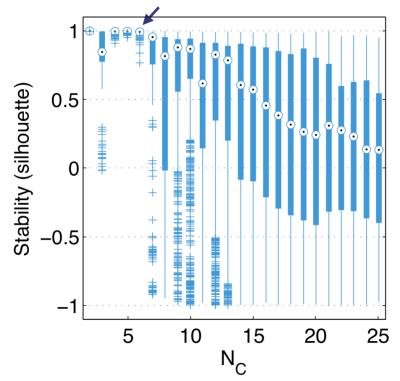
Upon clustering the feature set using GMM, we observe decreasing improvements in GMM fit quality, as assed using $$$AIC$$$, above $$$N_C = 6$$$ (Fig. 2) and we see that GMM performs robustly only when $$$N_C \leq 6$$$, when using silhouettes (Fig. 3). Contiguity z-scores remained high ($$$>3$$$) for nearly all tumours for all $$$N_C$$$. These results led us to select $$$N_C = 6$$$ as the recommended value for this data set.
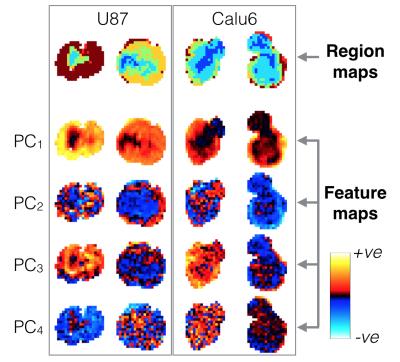
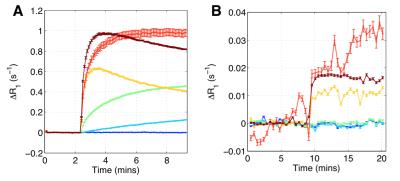
Fig. 4 shows tumour region maps from our recommended method for four representative tumours, alongside principal component feature maps. Region maps show spatially contiguous regions and a rim-core structure. Mean within-cluster enhancement curves in Fig. 5 show that GMM locates regions with distinct physiological characteristics, and with enhancement kinetics that are consistent with DCE-MRI and OE-MRI curves previously reported using manual region selection.[3,4]
Discussion
The rim-core structures present in region maps and the high contiguity z-scores, particularly with high $$$N_C$$$ values, suggest there is a biological basis to the regions identified. The recommended method locates regions where there is DCE-MRI but not OE-MRI enhancement (green and light blue regions/curves in figures 4 and 5). These have been shown to be relevant to hypoxia.[3,4] It is encouraging that in our current work, using a purely objective and data-driven methodology, we identify regions with these characteristics, removing the need for operator-based feature extraction.
When clustering data, we desire a high enough $$$N_C$$$ to identify features of potential importance (and in this case to adequately characterise tumour heterogeneity), whilst keeping a low enough $$$N_C$$$ to ensure the repeatability and reliability of our results. This lends importance to the range of evaluation metrics used in this study as they provide objective measures from which the optimal $$$N_C$$$ can be determined.
Conclusion
The methods presented here robustly locate functionally distinct regions within tumours. They require fewer a priori assumptions about the data than ad-hoc manual feature selection methods and provide further insight into intratumoural heterogeneity.Acknowledgements
This is a contribution from the CRUK & EPSRC Cancer Imaging Centre in Cambridge and Manchester (C8742/A18097).References
1: Alizadeh A A, et al. Toward understanding and exploiting tumor heterogeneity. Nat Med. 2015;21(8):846-53.
2: O’Connor J P B, et al. Imaging intratumor heterogeneity: role in therapy response, resistance, and clinical outcome. Clin Cancer Res. 2015;21(2):249-57.
3: Linnik I V, et al. Noninvasive tumor hypoxia measurement using magnetic resonance imaging in murine U87 glioma xenografts and in patients with glioblastoma. Magn Reson Med. 2014;71(5):1854-62.
4: O’Connor J P B, et al. Oxygen-enhanced MRI accurately identifies, quantifies, and maps tumor hypoxia in preclinical cancer models. Cancer Res. 2016;76(4):1-9.
5: Rousseeuw P J. Silhouettes: a graphical aid to the interpretation and validation of cluster analysis. J Comput Appl Math. 1987;20:53-65
Figures