0975
Quantitative characterization of brain tumor heterogeneity using diffusion basis spectrum imaging with extended isotropic spectrum (DBSI_EIS)1Washington University in St. Louis, St. Louis, MO, United States, 2University of Arizona, AZ, United States, 3The university of Alabama at Birmingham, Birmingham, AL, United States
Synopsis
Tumors are typically heterogeneous, and may contain different grades of tumor cells, different types of tumor cells, edema and/or abnormal vascular structures. A noninvasive, non-radioactive technique to provide multiple parametric and quantitative images for better profiling the heterogeneity of tumors is highly needed. We demonstrated that diffusion basis spectrum imaging with extended isotropic spectrum (DBSI-EIS) is capable to identify structural heterogeneity in brain tumor lesions, including various grades of tumor cells and perfusion, which make it a new and unique technique to clinically evaluate tumors for comprehensive diagnosis and accurate treatment evaluation.
Purpose
Primary brain tumors have heterogeneous pathology with tumor grade, tumor vessel density and invasiveness playing roles in patient management. Currently, the clinical monitoring of primary brain tumors is performed by contrast-enhanced MRI at standard intervals, but there are limitations in the conventional MRI assessment particularly in non-enhancing tumors. Advances in PET tracers targeting system L amino acid transport such as 3,4-dihydroxy-6-[18F]fluoro-L- phenylalanine (18F-FDOPA) 1 have shown promise for brain tumor imaging, particularly for defining gross tumor volume and tumor margins. In this study, adult patients with known or suspected brain gliomas that were non-enhancing or had substantial non-enhancing regions underwent simultaneous 18F-FDOPA-PET/MRI prior to planned standard-of-care surgical resection and/or stereotactic biopsy. We compared FDOPA-PET/MRI imaging features with WHO tumor grade. To better characterize the heterogeneity of the tumors for more accurate and complete diagnosis, treatment planning and post-treatment evaluation, we developed diffusion basis spectrum imaging with extended isotropic spectrum (DBSI-EIS), based on our previous success on DBSI 2,3. DBSI-EIS is a non-invasive imaging method customized for tumors characterization by providing the quantitative distributions of different grades of tumor cells and capillary blood perfusion within the tumor in a single clinical imaging scan.Methods
In this preliminary study, six participants with biopsy proved histologies drawn from the 18F-FDOPA PET/MRI study were processed with DBSI-EIS. The PET/MRI imaging were performed on a simultaneous 3.0 Tesla PET/MRI system, Siemens Biograph mMR scanner (Siemens Health Care, Erlangen, Germany). The MRI imaging protocol included standard clinical sequences, including a 3D T1 (MPRAGE, 1mm isotropic voxels), T2, FLAIR, SWI, dynamic susceptibility contrast (DSC) perfusion, and post gadolinium T1 weighted images. Dynamic PET imaging was acquired for at least 45 minutes and up to 60 minutes after the intravenous injection of 5 mCi of [18F] FDOPA. The emission data were collected with standard technique. Attenuation correction for PET/MRI studies was performed using the Dixon MRI sequence. The tumor ROI was selected based on FDOPA uptake which is 1.5 higher than the contralateral normal brain activity. Diffusion MRI images were collected (2x2x2mm voxels, TR/TE = 9500ms/93ms, multi-b-value scheme, 78 directions and bmax = 2000s/mm2). DBSI-EIS expanded the diffusion basis employed in original DBSI to model the capillary blood perfusion effects that have been used in intra-voxel incoherent motion imaging 4. By employing inverse regularization technique, DBSI-EIS cell fraction in each tumor grade and perfusion fraction were generated. The ADC cutoffs for tumor grade were chosen based on previous study 5 and our own data sets.Results
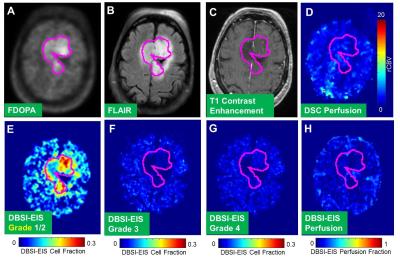
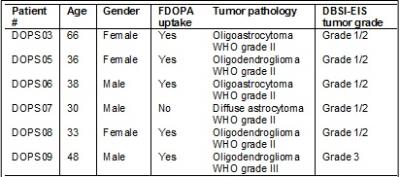
DBSI_EIS detected tumor grade that is consistent with that assessed by the biopsy pathology (Tab. 1) in five patients (Patients #DOPS03, DOPS05, DOPS06, DOPS08 and DOPS09). Interestingly, in one case (Patient # DOPS07), DBSI-EIS detected a Grade 1/2 tumor consistent with biopsy finding, while FDOPA PET did not show uptake above the normal brain in the tumor. A representative patient, DOPS03, previously underwent gross total resection 15yrs prior to FDOPA PET/MR imaging for recurrent tumor. Current biopsy results classified the tumor as an Oligodendroglioma WHO grade II with low proliferation (Ki-67 =4.7%), negative IDH-1, and 1p19q deletions. In Figure1, we found the tumor for this patient has substantial FDOPA uptake (Fig 1A, outlined in magenta) and matching FLAIR signal (Fig 1B). The lesion showed no T1 contrast enhancement (Fig 1C) and demonstrated lack of elevated perfusion based on the DSC T2*-based perfusion relative cerebral blood volume (rCBV) map (Fig 1D) and DBSI-EIS perfusion analysis (Fig 1H). DBSI-EIS generated images for Grade 1-2 tumor (Fig. 1E), Grade 3 tumor (Fig. 1F), and Grade 4 tumor (Fig. 1G). DBSI-EIS found that Grade 2 tumor cells dominated in this patient without higher grade component involvement.Discussion and Conclusion
This preliminary study demonstrated the capability of DBSI-EIS to noninvasively characterize the structural heterogeneity in brain tumors, including various grades of tumor cells and capillary blood perfusion within the tumors, consistent with pathology assessment on biopsy tissues. Our data suggest DBSI-EIS is a promising multi-parametric imaging technique to accurately measure cellularity and tumor grade but larger studies will be needed before definitive conclusions can be made about the role of this technique.Acknowledgements
Supported by Siteman Cancer Center and Barnes-Jewish Hospital Foundation and NMSS RG5265 A1.References
1. Beuthien-Baumann B, Bredow J, Burchert W, et al. 3-O-methyl-6-[18F]fluoro-L-DOPA and its evaluation in brain tumour imaging. Eur J Nucl Med Mol Imaging. 2003;30(7):1004-1008.
2. Wang Y, Wang Q, Haldar JP, et al. Quantification of increased cellularity during inflammatory demyelination. Brain. 2011;134(Pt 12):3590-3601.
3. Wang Y, Sun P, Wang Q, et al. Differentiation and quantification of inflammation, demyelination and axon injury or loss in multiple sclerosis. Brain. 2015;138(Pt 5):1223-1238.
4. Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161(2):401-407.
5. Yamasaki F, Kurisu K, Satoh K, et al. Apparent diffusion coefficient of human brain tumors at MR imaging. Radiology. 2005;235(3):985-991.
Figures