0966
Combined multi-band z-shimming using a novel auto-calibration routine1Institute of Neuroscience and Medicine, Medical Imaging Physics (INM-4), Forschungszentrum Juelich, Juelich, Germany, 2Faculty of Medicine, Department of Neurology, JARA, RWTH Aachen University, Aachen, Germany
Synopsis
EPI images suffer from signal dropouts and z-shimming is an effective technique to recover parts of the lost signal. Its inherent drawbacks are a scan time prolongation and the necessity to estimate subject-specific z-shim gradient moments. We introduce multi-band imaging to z-shimming to eliminate the scan time prolongation and propose a novel method to estimate optimal z-shim gradient moments. The results show less signal dropout in the z-shimmed images and prove our z-shim gradient moment estimation to be robust and efficient. Due to a reduced anatomical contrast, our multi-band z-shim approach might be most beneficial for BOLD-contrast based fMRI.
Purpose
EPI images suffer from signal dropouts largely caused by susceptibility-induced intra-voxel spin dephasing in the through-slice direction. Z-shimming techniques acquire additional images with a prephasing gradient applied in that direction and form a composite image with reduced signal dropout1. Though it is efficient regarding signal recovery, z-shimming entails an acquisition time penalty dominated by the number of acquired offsets and requires one to find subject-dependent prephasing gradient moments which are unknown before acquisition.
Reducing the TR for each z-shim image to maintain acquisition times limits brain coverage when using equal slice thicknesses. Conventional visual inspection of test scans to estimate suitable z-shim gradient moments introduces an operator-dependent bias. Addressing the problem of prolonged acquisition times, we suggest the combination of multi-band imaging2 and z-shimming to lower the achievable TR while preserving slice thickness and whole-brain coverage for the same acquisition times. Independent from this acquisition scheme, we introduce a novel auto-calibration routine based on a rapid pre-scan to automatically determine optimal subject-specific z-shim gradient moments.
Methods
We implemented the z-shim option into a multi-band EPI sequence. The z-shim gradient moment can be adjusted by a factor fz producing a prephasing gradient moment in the range of ±90% of the slice selection gradient. In multi-band mode the gain in acquisition speed is exploited to acquire each slice repeatedly with different z-shim prephasing moments. Hence, the effective TR for each slice is reduced by the applied multi-band factor and total sampling time of the composite multi-slice volume is maintained.
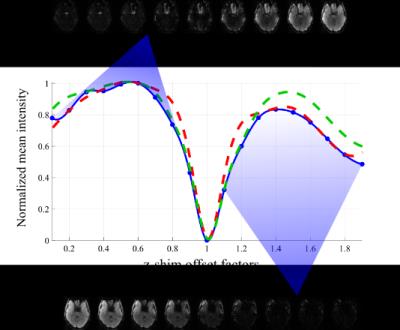
For our auto-calibration routine we postulate that additional z-shim offset images Iz are optimal when they have a high mean signal intensity in regions where the standard image I0 has low intensity. Consequently, we aim at maximizing the mean intensity in the difference image (Iz – I0) over all positive-valued image voxels. In auto-calibration mode the sequence is adjusted such that during each TR the fz-value is varied from its minimum to its maximum value in 10%-steps. The post-processing routine calculates the mean residual signal intensity for each offset image and quantifies the potential signal contribution of each z-shim factor. The most favourable z-shim offsets are derived from the resulting maximum residual intensity values.
Results
To evaluate the performance of our proposed methods in comparison to that of standard EPI, we used the following protocol on 3 healthy volunteers using a Siemens 3T Trio scanner with a 32-channel head coil.
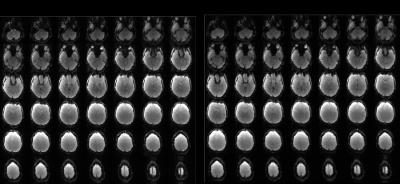
B0 maps were acquired using a multi-echo GRE sequence3 and standard EPI data was acquired using TR/TE=3000/30 ms, slices=42, slice thickness/gap=3/0 mm, FOV=210x210 mm2 and matrix size=70x70 providing whole-brain coverage with an isotropic 3x3x3 mm3 resolution. Results for both acquisitions are shown in Fig. 1 and reveal EPI dropouts in regions of extreme B0 offsets.
For the auto-calibration we acquired 19 offset images using the same parameters as for the EPI and performed the post-processing to obtain the optimal fz-values. Using 3-fold multi-band acceleration to reduce TR to 1000 ms, the time for acquisition and processing can be as short as 30 sec. Given that the B0 offsets vary from negative values in the inferior brain to positive values near the sinus and auditory canals, we determined one optimal z-shim factor per B0 offset polarity. This is in agreement with Fig. 2, which shows the results of applying the mean intensity analysis for all volunteers and revealing signal recovery peaks for distinct positive and negative offsets.
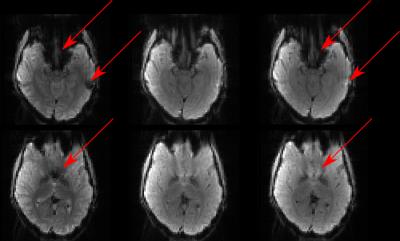
Another dataset was acquired using the same 3-fold multi-band EPI sequence with the determined z-shim factors. To investigate the effect of including further z-shim offset images we also calculated a composite image from all 19 prephasing settings and compared it to the results of our fz-optimized setting. Images for our fz-optimized and the 19-offset z-shimmed EPI are shown in Fig. 3 for the entire volume and two representative slices are depicted in Fig. 4. It was observed that signal was significantly recovered using z-shimming. Furthermore, results from the optimized offsets were shown to be comparable to those from the 19 offsets.
Discussion and conclusions
The multi-band z-shimming approach is capable of reducing signal dropout without compromising acquisition time or volume coverage. In contrast to a similar volume selective z-shimming approach4, no signal variation caused by varying TR across slices is introduced and the z-shim effect is available in the entire volume. Due to the reduced signal dropout, our approach might be beneficial for fMRI in brain regions with strong B0 offsets. The proposed auto-calibration is robust and easy to implement. It produces near-optimal results with a low number of offsets.Acknowledgements
No acknowledgement found.References
1. Frahm J, Merboldt K-D, Hänicke W. Direct FLASH MR imaging of magnetic field inhomogeneities by gradient compensation. Magn Reson Med. 1988;6(4):474-480.
2. Setsompop K, Gagoski BA, Polimeni J R, et al. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn Reson Med. 2012;67(5):1210-1224.
3. Hetherington HP, Chu WJ, Gonen O, et al. Robust fully automated shimming of the human brain for high-field 1H spectroscopic imaging. Magn Reson Med. 2006;56(1):26-33.
4. Du YP, Dalwani M, Wylie K, et al. Reducing susceptibility artifacts in fMRI using volume-selective z-shim compensation. Magn Reson Med. 2007;57(2):396-404.
Figures