0964
Real-time shimming of the human spinal cord using a 24-channel shim array coilRyan Topfer1, Alexandru Foias1, Nikola Stikov1,2, and Julien Cohen-Adad1,3
1NeuroPoly Lab, Institute of Biomedical Engineering, Polytechnique Montreal, Montreal, QC, Canada, 2Montreal Heart Institute, Université de Montréal, Montreal, QC, Canada, 3Functional Neuroimaging Unit, CRIUGM, Université de Montréal, Montreal, QC, Canada
Synopsis
Pathologies of the spinal cord are a primary cause of functional disability and chronic pain. Although MRI already plays a role in the evaluation of these pathologies, it continues to be hampered by artifacts due to magnetic field inhomogeneity. This study reports the first results from applying a specially designed 24-channel shim array to compensate respiration-induced magnetic field inhomogeneity in the human spinal cord in real-time. This approach has the potential to improve the quality of EPI and spectroscopy in the spinal cord.
Introduction
The sensitivity of MRI to magnetic susceptibility is both a blessing and a curse: While small-scale changes in the magnetic field permit useful image contrasts, such as the blood-oxygenation-level dependent (BOLD) effect, larger scale field distortions, such as those due to air-tissue interfaces, can cause image artifacts such as signal loss in gradient-echo (GRE) imaging and geometric distortion in echo-planar imaging (EPI). The proximity of the spinal cord to the lungs – the body’s most extensive internal air-tissue interface – makes it particularly susceptible to these field effects, which generally vary in time, along with respiration (e.g., Figure 1).1–3 Given the extent of these artifacts, along with the inherent subtlety of the signal changes associated with BOLD activation (about 1%), fMRI of the spinal cord remains technically challenging, as underscored in a recent review: “respiration-induced change in B0 is one of the main reasons very few people dare to venture into fMRI experiments below the cervical level.”4 This study presents the first results updating shims in real-time to correct for respiration-induced distortions in the spinal cord.Methods
Real-time shimming hardware is exhibited in Figure 2.5 Calculation of the shim updates was informed by the work of Van Gelderen et al.6 which suggested a possible linear relation between the time-varying magnetic field and the respiratory cycle as tracked by a pressure reading from respiratory bellows strapped to the subject’s abdomen: $$$ \frac{ \partial b_{\chi} (\textbf{r}, t) }{ \partial t } = c_{\chi}(\textbf{r}) \frac{ \partial p(t) }{ \partial t } $$$, which, when integrated, yields $$$ b_{\chi} (\textbf{r}, t) = c_{\chi}(\textbf{r})p(t) + b_{\chi|o}(\textbf{r}) $$$. It follows that the coupling coefficients $$$c_{\chi}(\bf{r})$$$ and the static field offset $$$b_{\chi|o}(\textbf{r})$$$ might be estimated by GRE field maps acquired in as few as two distinct apneas—a sort of calibration procedure by which all future field perturbations can be inferred based on the associated pressure reading (Figure 3).Experiments: Six healthy subjects were scanned on a 3 T system (Siemens TIM Trio). Standard 2nd-order static shimming was first performed using the MRI shims. To calibrate the real-time shim updates, GRE field maps were acquired in inspired and expired respiratory states (e.g. Figures 1 and 3) with acquisition and phase processing parameters similar to those described in a previous work.5 To demonstrate the utility of real-time shimming, a 2D sagittal GRE-EPI sequence typical of fMRI was performed during normal respiration, with and without real-time shimming, for which shim updates were issued every 250 ms based on the respiratory trace sampled at 100 Hz. The EPI sequence used the following parameters: TE=15 ms, TR=500 ms; flip angle 70°; bandwidth=1502 Hz/pixel; R=2 acceleration factor using GRAPPA; partial Fourier=6/8; spatial resolution=1.5x1.5 mm2 in-plane, with 7 sagittal slices of thickness 5.0 mm, for an effective FOV=35x156x192 mm3; 100 image volumes were acquired, for a total acquisition time of 50 s. The temporal signal-to-noise ratio (tSNR) of the EPI data was calculated as the average voxel intensity across the time series divided by its standard deviation.
Results and Discussion
Over the spinal cord, real-time shimming improved the mean EPI tSNR by 18.9 +/- 13.2 %, while increasing tSNR over the entire region considered in the shim optimization by 12.2 +/- 10.1%. Figure 4 summarizes these improvements and Figure 5 shows visual results for the first two subjects.This study demonstrates the benefit of real-time shimming for spinal cord EPI. Regarding improvements to tSNR, a considerable intersubject variability was observed likely due to anatomical differences that determine the extent of respiration-induced distortion. Though increased tSNR was observed across subjects, the real-time protocol could nevertheless be improved, for example, by updating the shims more frequently, and by accounting for transmission delays. Furthermore, apart from ignoring other contributions to the time-varying magnetic field (e.g. eddy currents, cardiac pulsation), the validity of the linear pressure-to-field relation used to update the shims has been taken for granted; other models of the time-evolving field,7 or means of tracking it,8–10 may ultimately prove more appropriate.
In addition to signal enhancement and artifact reduction for EPI, real-time shimming stands to benefit T2*-weighted imaging generally, as well as spectroscopic studies of the spinal cord.
Acknowledgements
We thank the team at Resonance Research Inc. – Piotr Starewicz, Kai-Ming Lo, Karl Metzemaekers, and Donald Jette – for their work constructing the shim hardware. Funded by the Canada Research Chair in Quantitative Magnetic Resonance Imaging (JCA), the Canada Foundation for Innovation [32454], the Canadian Institutes of Health Research [CIHR FDN-143263], the Fonds de Recherche du Québec - Santé [28826], the Fonds de Recherche du Québec - Nature et Technologies [2015-PR-182754], the Natural Sciences and Engineering Research Council of Canada [435897-2013], the Quebec BioImaging Network, and Polytechnique MEDITIS.References
1. Marques, J. P. & Bowtell, R. Application of a Fourier-based method for rapid calculation of field inhomogeneity due to spatial variation of magnetic susceptibility. Concepts Magn Reson Part B Magn Reson Eng 25B, 65–78 (2005).2. Verma, T. & Cohen-Adad, J. Effect of respiration on the B0 field in the human spinal cord at 3T. Magn Reson Med 72, 1629–36 (2014).
3. Vannesjo, S. J. et al. Breathing-induced B0 field fluctuations in the cervical spinal cord at 7T. in Proceedings of the 24th Annual Meeting of ISMRM, Singapore, 2016. Abstract 49.
4. Cohen-Adad, J. Functional MRI of the Spinal Cord: Current Status and Future Developments. Semin. Ultrasound, CT MRI 5675–5679 (2016). doi:10.1053/j.sult.2016.07.007
5. Topfer, R. et al. A 24-channel shim array for the human spinal cord: design, evaluation and application. Magn Reson Med (2016). doi:10.1002/mrm.26354
6. van Gelderen, P., de Zwart, J. A., Starewicz, P., Hinks, R. S. & Duyn, J. H. Real-time shimming to compensate for respiration-induced B0 fluctuations. Magn Reson Med 57, 362–8 (2007).
7. Bianciardi, M. et al. Evaluation of dynamic off-resonance correction of respiratory instability in MRI signals for high-order spherical harmonic basis set and multivariate modeling of respiratory sources. in Proceedings of the 22nd Annual Meeting of ISMRM, Milan, 2014. Abstract 1623.
8. Boer, V. O., van de Bank, B. L., van Vliet, G., Luijten, P. R. & Klomp, D. W. J. Direct B0 field monitoring and real-time B0 field updating in the human breast at 7 Tesla. Magn Reson Med 67, 586–91 (2012).
9. Haeberlin, M. et al. Real-time motion correction using gradient tones and head-mounted NMR field probes. Magn Reson Med 74, 647–660 (2015).
10. Duerst, Y. et al. Utility of real-time field control in T2*-Weighted head MRI at 7T. Magn Reson Med 76, 430–439 (2016).
Figures
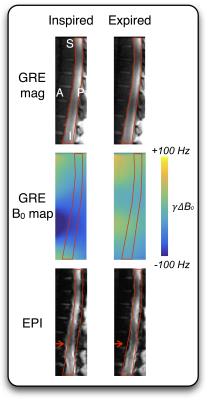
Figure 1: Respiration-induced
distortion in the thoracic spine of one subject. Subjects were asked to inspire
naturally (left column) and maintain the apnea for the GRE field mapping scan
(top/middle row: magnitude/field map) and to repeat the breath hold for the EPI
(bottom row). The procedure was then repeated in an expired state (right
column). The spinal canal was traced on the “inspired” GRE magnitude and superposed
onto the other images (red contours). The top row demonstrates that no displacement
of the spinal cord occurs between respiratory states, yet the EPI exhibits an
apparent motion along the phase-encode direction (arrows).
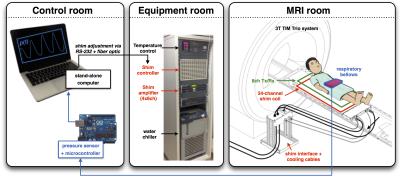
Figure 2: System overview. The
shim array consists of 24 independently driven electrical coils, atop which
sits a custom-built 8-channel transceiver. The two coil arrays insert into the
patient bed-table so as to lie in close proximity to the subject’s spine. To
monitor patient respiration in real-time, an air-filled latex bladder (i.e.
respiratory bellows, strapped to the subject’s abdomen) was linked by a 15 m
length of vinyl tubing to a pressure-sensing transducer and Arduino
microcontroller. The microcontroller feeds pressure measurements into a
stand-alone computer running Matlab, which then issues the associated shim
currents to the shim controller.
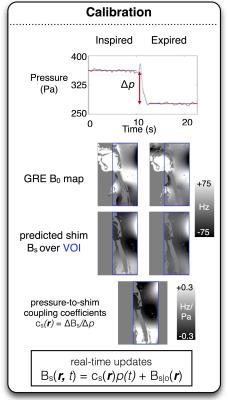
Figure 3: Demonstration
of the real-time shimming calibration procedure. GRE field maps are acquired
under inspired and expired conditions and the optimal shim currents for the
24-channel array are calculated, taking into account the non-linear constraints
of the amplifier.
The
difference between the predicted shim fields for the two apneas is divided by
the difference in the associated pressures to yield the optimal
pressure-to-shim field coupling coefficients cs(r).
In
practice, real-time updates to shim currents are reduced to simply multiplying
the scalar pressure reading by a vector and adding to it a vector of pressure-independent
DC offset currents.
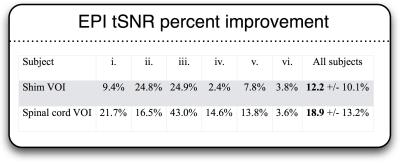
Figure 4: Mean improvements to
EPI tSNR for all subjects across two volumes of interest (VOI): the entire
region considered in the shim optimization (“Shim VOI”), and a smaller region
consisting of the segmented spinal cord (“Spinal cord VOI”). Across subjects,
the mean tSNR increase was 12.2 % and 18.9 % for the respective VOIs. The
improvement was statistically significant at the P=0.05 significance
level for the Shim VOI, and at the level of P=0.01 for the spinal cord
VOI (bilateral paired Student’s t-test).
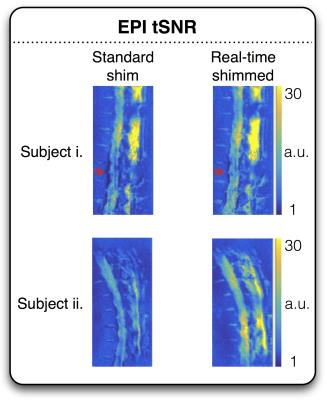
Figure 5: Improved EPI tSNR
with real-time shimming for two subjects. The top row features the same subject
as Figure 1 with red arrows again pointing to the region of marked
respiration-induced distortion along the spinal cord. EPI signal stability in
the region is significantly improved by real-time shimming.