0951
Validation of contralateral parenchymal enhancement on DCE-MRI as a biomarker of survival in patients with ER-positive/HER2-negative breast cancer1Image Sciences Institute, University Medical Center Utrecht, Utrecht, Netherlands, 2Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 3Unità di Radiologia, IRCCS Policlinico San Donato, Milan, Italy, 4Department of Radiology, University Medical Center Utrecht, Utrecht, Netherlands
Synopsis
This study was performed to validate whether MR contrast-enhancement in stromal tissue of the disease-free breast is related to the survival of patients with cancer in the other breast. A recent study in 398 patients with estrogen-receptor positive and human-epidermal-growth-factor-2 negative invasive breast cancer showed that more pronounced contralateral parenchymal enhancement (CPE) was associated with improved patient survival. In this study, we extracted CPE to re-test the finding in a comparable patient population from an independent cancer center. In 287 patients, CPE reproduces as a biomarker for long-term survival. This reproducible imaging finding has potential towards the personalization of care.
Purpose
This study was performed to validate whether the contrast-enhancement on magnetic resonance imaging (MRI) in stromal tissue of the disease-free breast is related to the survival of patients with cancer in the other breast. Perfusion of the stroma surrounding a tumor on dynamic contrast-enhanced MRI has been associated with patient survival after neoadjuvant chemotherapy.1,2 The properties of this surrounding stroma could, however, be tumor-induced. Assuming a common microenvironment between both breasts, we hypothesized that the stroma in the disease-free breast (i.e., contralateral to known cancer) is comparable to that in the diseased breast before tumorigenesis. Therefore, by analyzing the stroma in the disease-free breast before treatment, we might gain insight into the role of the breasts’ microenvironment on patient outcome, possibly establishing a predictive imaging biomarker. In a recent study in patients with estrogen-receptor positive and human-epidermal-growth-factor-2 negative (ER+/HER2-) breast cancer, we found that more pronounced contralateral parenchymal enhancement (CPE) was associated with improved patient survival.3 The purpose of this study was to validate CPE as a biomarker for patient survival in an independent patient cohort from a different cancer institution.Methods
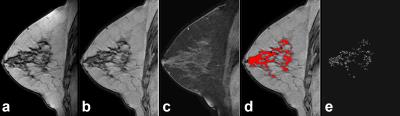
CPE, as a possible biomarker, was established in a test cohort of 398 patients with ER+HER2- breast cancer from Cancer Center #1 (biomarker-discovery set).3 In this study, we extracted CPE to re-test the finding in a comparable patient population from Cancer Center #2 (validation set). Breast cancer survival between the biomarker-discovery and the validation sets were compared. This retrospective Health Insurance Portability and Accountability Act (HIPAA)-compliant study received Institutional Review Board (IRB) approval. We identified 287 consecutive patients with pathology-proven unilateral, early stage ER+/HER2- invasive ductal carcinoma treated at Cancer Center #2 between 2005 and 2009. MRI was performed using a General Electric 1.5T unit and 8-channel breast coil. MRI sequences used in this analysis were sagittal: 1) non-fat-suppressed T1-weighted images and 2) fat-suppressed T1-weighted images acquired before and at three time points (temporal resolution: 120s) after the administration of intravenous gadolinium (0.2mmol/kg). Using the non-fat-suppressed T1-weighted images the parenchymal tissue of the disease-free breast was segmented adopting the previously published method.3 In short, the disease-free breast was automatically segmented in 3D, field inhomogeneities were corrected, after which the parenchymal tissue was segmented.4,5,6 The post-contrast images were registered to the pre-contrast images using deformable registration to compensate for patient motion.7 The late parenchymal enhancement was calculated using (S(t3)-S(t1))/S(t1), where S(t1) is the signal at the first post-contrast scan and S(t3) at the third post-contrast scan (Figure 1). The average of the top-10% most enhancing voxels was defined as contralateral parenchymal enhancement (CPE). This is a unitless quantitative value that can be compared between patients. Differences in patient and tumor characteristics between the biomarker-discovery3 (Cancer Center #1) and validation set (Cancer Center #2) were tested using Kruskal-Wallis tests for continuous variables and Fisher’s exact tests for categorical variables. Analysis was performed for overall survival (OS) and invasive disease-free survival (IDFS).8 Cox proportional hazard models and Kaplan-Meier estimators were used to investigate the relation between CPE and OS and IDFS.Results
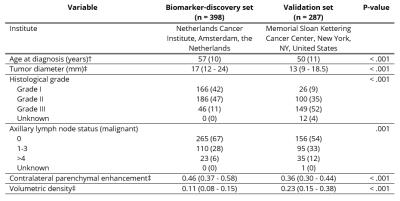
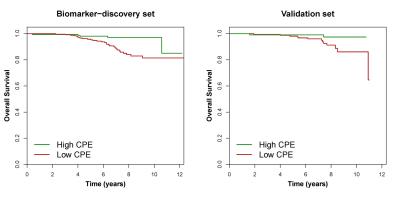
Patient and tumor characteristics are described in Table 1, MRI protocols in Table 2. Age at diagnosis (P < .001), tumor diameter (P < .001), histological grade (P < .001), and the number of axillary lymph nodes positive for malignancy (P = .001) were significantly different between the biomarker-discovery and validation sets (Table 1). The median time to follow-up was 85 months (interquartile range 68–108 months) in the biomarker-discovery set and 88 months (interquartile range 76–102 months) in the validation set (P = .62). The two sets were independently dichotomized. Patients with high CPE had a better OS and IDFS in both the biomarker-discovery set (OS: Hazard Ratio (95%CI) = 0.36 (0.14-0.93), P = .036; IDFS: Hazard Ratio (95%CI) = 0.58 (0.30-1.11), P = .099) and the validation set (OS: Hazard Ratio (95%CI) = 0.22 (0.05-0.99), P = .049; IDFS: Hazard Ratio (95%CI) = 0.32 (0.14-0.72), P = .007). Kaplan-Meier estimators for breast cancer overall survival are shown in Figure 2.Discussion
This study confirms that higher perfusion of the stroma of the non-diseased breast on DCE-MRI of women with breast cancer is associated with improved overall and disease-free survival. This biomarker was validated at two different cancer centers, giving similar conclusions despite differences in patient population and MRI protocols.Conclusion
Contralateral parenchymal enhancement reproduces as a biomarker for long-term survival in patients with unilateral early-stage ER-positive/HER2-negative invasive ductal carcinoma. This reproducible imaging finding has the potential to be both predictive and prognostic contributing towards the personalization of care.Acknowledgements
No acknowledgement found.References
1. Hattangadi J, Park C, Rembert J, et al. Breast stromal enhancement on MRI is associated with response to neoadjuvant chemotherapy. Am J Roentgenol 2008; 190:1630-1636.
2. Jones EF, Sinha SP, Newitt DC, et al. MRI Enhancement in Stromal Tissue Surrounding Breast Tumors: Association with Recurrence Free Survival following Neoadjuvant Chemotherapy. PloS one 2013; 8:e61969.
3. van der Velden BH, Dmitriev I, Loo CE, Pijnappel RM, Gilhuijs KG. Association between parenchymal enhancement of the contralateral breast in dynamic contrast-enhanced MR imaging and outcome of patients with unilateral invasive breast cancer. Radiology 2015; 276:675-685.
4. Gilhuijs KG, Deurloo EE, Muller SH, Peterse JL, Schultze Kool LJ. Breast MR Imaging in Women at Increased Lifetime Risk of Breast Cancer: Clinical System for Computerized Assessment of Breast Lesions Initial Results. Radiology 2002; 225:907-916.
5. Tustison NJ, Avants BB, Cook PA, et al. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging 2010; 29:1310-1320.
6. Klifa C, Carballido-Gamio J, Wilmes L, et al. Quantification of breast tissue index from MR data using fuzzy clustering. 1 ed: Conf. Proc. IEEE Eng. Med. Biol. Soc, 2004; 1667-1670.
7. Dmitriev ID, Loo CE, Vogel WV, Pengel KE, Gilhuijs KGA. Fully automated deformable registration of breast DCE-MRI and PET/CT. Phys Med Biol 2013; 58:1221-1233.
8. Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol 2007; 25:2127-2132.
Figures