Mami Iima1,2, Masako Kataoka1, Shotaro Kanao1, Natsuko Onishi1, Makiko Kawai1, Akane Ohashi1, Rena Sakaguchi1, Ayami Ohno Kishimoto1, Masakazu Toi3, and Kaori Togashi1
1Department of Diagnostic Imaging and Nuclear Medicine, Graduate School of Medicine, Kyoto University, Kyoto, Japan, 2Hakubi Center for Advanced Research, Kyoto University, Kyoto, Japan, 3Department of Breast Surgery, Graduate School of Medicine, Kyoto University, Kyoto, Japan
Synopsis
The association of IVIM/non-Gaussian
diffusion MRI parameters with biological feature or subtypes in breast cancer
was evaluated. For 144 malignant lesions, IVIM (fIVIM,D*) and non-Gaussian
diffusion (ADCo,K) parameters were estimated from DWI series with 16 b values
(0-2500sec/mm2), as well as syntheticADC (sADC) (b=200,1500sec/mm2) and ADC
(b=0,800sec/mm2). sADC and K values were significantly different between
ER,PgR,andHer2 status (p<0.05,0.01,0.05 for sADC and p<0.05,0.05,0.05 for
K). There was a significant difference of ADC values between PgRandHer2 status
(p<0.05,0.01). No significant difference of IVIM was found. ADCo, sADC,
and ADC showed the statistical significance in differentiating subtypes of
breast cancer (p<0.05,<0.01,<0.01).
Introduction
Diffusion
MRI and apparent diffusion coefficient (ADC) widely used in breast MRI (1) are
based on monoexponential model. Non-Gaussian diffusion parameters are
relatively new DWI-derived parameters providing important information on tissue
microstructure beyond ADC (2,3). However, their accurate estimation requires
time-consuming fitting of the DWI signal with multiple b values using
biophysical models (e.g.; kurtosis model) with long acquisition time. To reduce
acquisition and processing times, we have recently introduced a “synthetic ADC”
(sADC) derived from the signal acquired at a few “key b values”, which intrinsically
include both non-Gaussian diffusion and IVIM effects, while maximizing information
on tissue structure, without actually estimating diffusion parameters (4). We evaluated
if sADC as well as IVIM and quantitative non-Gaussian diffusion MRI parameters can
be used to differentiate biological features or subtypes in breast cancer.Materials and Methods
405 patients were prospectively
enrolled and 144 malignant lesions were included in this study. Breast MRI was performed using a 3-T
system (Trio, B17; Siemens Healthcare) equipped with a dedicated
16-channel breast array coil. The following DWI (WIP) images were obtained: single shot
EPI with 16 b values of 0-2500 sec/mm2; repetition time/echo time, 4,600/86
ms; field of view, 160×300 mm2;
matrix, 80×166; slice
thickness, 3.0 mm; and acquisition time, 3min55s; The algorithm was implemented
in Matlab (Mathwork, Natick, MA) and comprised the following steps:
1/The signal acquired with b>200 s/mm²
was fitted using the kurtosis diffusion model to estimate ADCo and K:
S/So = {exp[-bADCo + K(bADCo)²/6]+NCF}1/2 [1]
where S0 is the theoretical
signal acquired at b=0, ADCo the virtual ADC which would be obtained
when b approaches 0, K the kurtosis parameter and NCF (noise correction factor)
a parameter which characterizes the “intrinsic” non-Gaussian noise contribution
within the images (3).
2/Then, the
fitted diffusion signal component was subtracted from the corrected raw signal
acquired with b<200s/mm² and the remaining signal was fitted using the IVIM
model to get estimates of the (T1,T2-weighted) flowing blood fraction, fIVIM,
and the pseudodiffusion, D* (3).
Additionally
a synthetic ADC encompassing both Gaussian and non-Gaussian diffusion effects
(4), sADC200-1500, was defined using only 2 b values as:
sADC200-1500 = ln
[Sn(b200)/Sn(b1500)]/1300 [2]
ADC0-800 was also defined using standard
monoexponential ADC model. IVIM and non-Gaussian diffusion parameters in the status of histological biomarkers (ER, PgR,
Her2, Ki-67) were compared using Mann-Whitney test. IVIM and non-Gaussian diffusion
parameters in different breast cancer subtypes (Her2-positive(H+), Luminal A(LA), Luminal B and
Her2-negative (LBH-), Luminal B and Her2- positive (LBH+), and Triple Negative (TN)) were
compared using Kruskal–Wallis test with a post-hoc
analysis.
Results
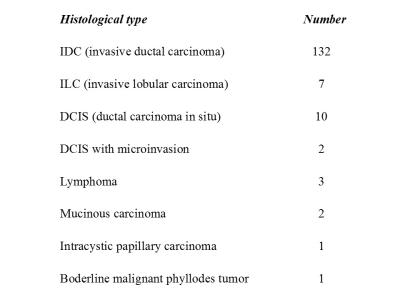
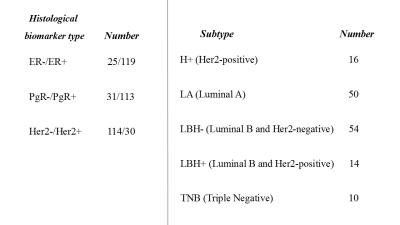
Patient characteristics are summarized in Fig.1and 2. Comparison of non-Gaussian diffusion MRI parameters with the breast cancer
biomarker and subtype status are shown in Fig 3 and 4. ER
and PgR positive tumors showed significantly lower sADC value (p <
0.05 and 0.01) and higher K value (p< 0.05 and 0.05). PgR positive tumors had significantly
lower ADC value (p < 0.05).Significantly higher sADC, ADC and lower K values
were noted in Her2 positive status (p
= 0.013, < 0.01,< 0.05). There was no significant difference of IVIM
parameters depending on the histological biomarker status. ADCo (p<0.05), sADC (p<0.01), and ADC (p<0.01)
showed the statistical significance in differentiating subtypes of breast
cancer as shown in Fig 4. Representative non-Gaussian
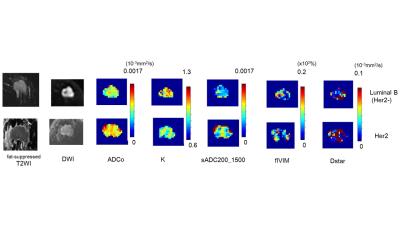
diffusion MRI and IVIM parameters maps are shown in Fig 5. The difference of ADCo, K and sADC
values between Luminal B (Her2 negative) and Her2 postive breast cancers are
remarakable.
Discussion
Only
sADC and K, not ADC, showed the significant difference with ER status in our study. This indicates
that non-Gaussian DWI parameters might provide additional information on the histological
biomarkers. Lower ADC value in ER positive tumors was found as in the
literature (5-7). Her2 positive tumors showed higher sADC or ADC values than
Her2 negative, as in the previous studies (9, 10). There was significant
difference of K values between positive and negative ER, PgR and Her2 status,
and this has not been reported elsewhere. Luminal B and Her2 negative cancer showed the smaller ADCo,
sADC and ADC values than luminal A, which was in agreement with the literature
(11).The combination of these Gaussian and Non-Gaussian diffusion parameters,
or synthetic ADC, might be a surrogate biomarker of the receptors expression as
well as subtypes of breast cancer. Conclusion
Non-Gaussian
diffusion parameters, especially sADC and Kurtosis, showed a good correlation
with biological factors and subtypes of breast cancer. There parameters might
provide useful information in identification of breast cancer biological factors and molecular subtypes without the need for contrast agents.Acknowledgements
This work was supported
by Hakubi Project of Kyoto University and MEXT KAKENHI Grant No. 15K19786.
The authors would like to thank Dr. Thorsten
Feiweier from Siemens Healthcare for providing WIP sequence.
References
1. Partridge
S, DeMartini W, Kurland B, et al. Differential diagnosis of mammographically
and clinically occult breast lesions on diffusion-weighted MRI. Journal of
Magnetic Resonance Imaging. 2010;31:562-570
2. Le
Bihan D et al. Diffusion Magnetic Resonance Imaging: What Water Tells Us about
Biological Tissues. PLoS Biol. 2015 Jul; 13(7): e1002203
3. Iima
M et al. Quantitative Non-Gaussian Diffusion and Intravoxel Incoherent Motion
Magnetic Resonance Imaging: Differentiation of Malignant and Benign Breast
Lesions. Investigative Radiology 2015:50:205-11
4. Iima
M et al. Clinical Intravoxel Incoherent Motion and Diffusion MR Imaging: Past,
Present and Future. Radiology 2016;278:1
5. Jeh
SK, Kim SH, Kim HS, et al. Correlation of the apparent diffusion coefficient
value and dynamic magnetic resonance imaging findings with prognostic factors
in invasive ductal carcinoma. J Magn Reson Imaging. 2011;33(1):102-9
6. Kamitani T, Matsuo Y, Yabuuchi H, et
al. Correlations between Apparent Diffusion Coefficient
Values and Prognostic Factors of Breast Cancer. Magnetic Resonance in Medical
Sciences. 2013;12(3):193-9
7. Choi
SY, Chang YW, Park HJ, Kim HJ, Hong SS, Seo DY. Correlation of the apparent
diffusion coefficiency values on diffusion-weighted imaging with prognostic
factors for breast cancer. The British Journal of Radiology.
2012;85(1016):e474-e9
8. Black
R, Prescott R, Bers K, Hawkins A, Stewart H, Forrest P. Tumour cellularity,
oestrogen receptors and prognosis in breast cancer. Clinical oncology.
1983;9(4):311-8
9. Kim
EJ, Kim SH, Park GE, et al. Histogram analysis of apparent diffusion
coefficient at 3.0 t: Correlation with prognostic factors and subtypes of
invasive ductal carcinoma. Journal of Magnetic Resonance Imaging.
2015;42(6):1666-78
10. Martincich
L, Deantoni V, Bertotto I, et al. Correlations between diffusion-weighted
imaging and breast cancer biomarkers. European radiology. 2012;22(7):1519-28
11. Kato F et al. Differences in morphological features and minimum apparent diffusion
coefficient values among breast cancer subtypes using 3-tesla MRI. Eur J Radiol. 2016
Jan;85:96-102