0946
Complementary value of contralateral parenchymal enhancement on DCE-MRI to conventional prognostic models and molecular assays of breast cancer1Image Sciences Institute, University Medical Center Utrecht, Utrecht, Netherlands, 2Division of Molecular Carcinogenesis, the Netherlands Cancer Institute–Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands, 3Department of Radiology, the Netherlands Cancer Institute–Antoni van Leeuwenhoek Hospital, Amsterdam, Netherlands
Synopsis
The purpose of this study was to assess whether MR contrast-enhancement in healthy stromal tissue of the breast is able to further stratify survival of patients considered to be at high risk according to prognostic models derived from the tumor. In 415 patients with pathology proven unilateral invasive ER+HER2- breast cancer, the contralateral parenchymal enhancement was automatically extracted. Contralateral parenchymal enhancement appears to complement existing prognostic models derived from the tumor. In patients at high risk according to conventional prognostic models or molecular assays, contralateral parenchymal enhancement was able identify a subgroup with a relative good survival.
Purpose
The purpose of this study was to assess whether MR contrast-enhancement in healthy stromal tissue of the breast is able to further stratify survival of patients considered to be at high risk according to prognostic models derived from the tumor. Patient and tumor markers are often used in models to select chemotherapy.1 Further individualization of chemotherapy has been accomplished using molecular assays such as Mammaprint and Oncotype DX, which have been shown to complement conventional prognostic models.2-5 While these tests assess the tumor, studies on breast stroma are relatively underexposed. Nonetheless, the stroma may play an important role. For example, perfusion of the stroma surrounding the tumor on dynamic contrast-enhanced MRI has been associated with patient survival.6,7 The properties of this surrounding stroma might, however, be induced by the tumor. We recently found that pronounced contrast-enhancement of the stroma in the unaffected healthy (i.e. contralateral) breast on MRI has been associated with superior survival of patients with estrogen-receptor positive/human-epidermal-growth-factor-2 negative (ER+HER2-) breast cancer.8 Given the interaction between tumor and stroma, it is likely that markers of survival derived from the tumor may be complemented with those from the healthy stroma. Therefore, the aim of this study was to determine whether contralateral parenchymal enhancement was able to select a subgroup of patients at low risk of death from the group considered to be at high risk according to routine prognostic models.Methods
After approval of the institutional review board and written informed patient consent, 415 patients with pathology proven unilateral invasive ER+HER2- breast cancer were consecutively included for a retrospective analysis. The patients participated in the MARGINS study (2000–2008), were eligible for breast conserving therapy, and were consecutively recruited for an additional preoperative breast MRI. MR images were acquired using a Siemens 1.5 T scanner. An unenhanced T1-weighted image was acquired, and after injection of gadolinium containing contrast four consecutive post-contrast series were acquired 90 s apart. The imaging parameters were: repetition time/echo time: 8.1/4.0 ms, flip angle: 20°, voxel size: 1.35 × 1.35 × 1.35 mm3.
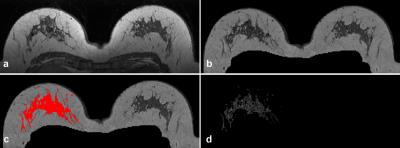
On the MR images, the contralateral stromal tissue was segmented using the previously published method(Figure 1).8 The post-contrast images were registered to the pre-contrast images using deformable registration to compensate for patient motion.9 The late parenchymal enhancement was calculated using (S(t4)- S(t1)) / S(t1), where S(t1) was the signal at the first post-contrast scan and S(t4) at the last post-contrast scan. The average of the top-10% most enhancing voxels was defined as contralateral parenchymal enhancement (CPE). This is an unitless value that can be compared between patients. Analysis was performed for overall survival (OS) in patients at high risk according to four existing prognostic models: the Nottingham Prognostic Index (NPI, where NPI > 3.4 is considered high risk); the Dutch guidelines for chemotherapy (Table 1); the 70-gene signature; and the 21-gene signature.1,3,4,10 For each population, CPE was dichotomized on the median. Kaplan-Meier estimators and log-rank tests were used to investigate the relation between CPE and OS.
Results
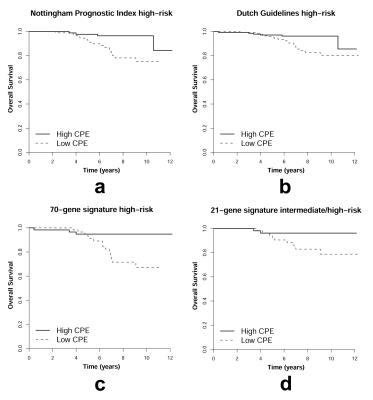
The average patient age was 57 years ± 10 years, the median tumor diameter on MRI was 17 mm (interquartile range: 12–25 mm). The median time to follow up was 85 months (interquartile range: 69–108 months), 35/415 (8%) patients succumbed. An NPI larger than 3.4 was found in 182/415 (44%) patients (21 succumbed, 12%). According to Dutch guidelines 283/415 (68%) patients were indicated for chemotherapy (25 succumbed, 9%). Sufficient frozen tumor tissue was available of 227/415 (55%) patients. A high-risk 70-gene signature was found in 116/227 (51%) patients (17 succumbed, 15%), an intermediate or high-risk 21-gene signature in 107/227 (47%) patients (11 succumbed, 10%). CPE significantly split survival in all subgroups at high risk (Figure 2, P=.002, P=.006, P=.004, and P=.043 for patients at high risk according to NPI, the Dutch guidelines, the 70-gene signature, and the 21-gene signature, respectively).Discussion
CPE on dynamic contrast-enhanced MRI appears to complement existing prognostic models derived from the tumor. In patients at high risk according to conventional prognostic models or molecular assays, CPE was able identify a subgroup with a relative good survival. This makes it tempting to hypothesize that CPE might be a biomarker that can be used to even further individualize therapy.Conclusion
In patients at high risk according to prognostic models derived from the tumor, MR contrast-enhancement in healthy stromal tissue of the breast can further stratify survival.Acknowledgements
No acknowledgement found.References
1. Galea MH, Blamey RW, Elston CE, Ellis IO. The Nottingham Prognostic Index in primary breast cancer. Breast Cancer Res Treat 1992; 22:207-219.
2. Cardoso F, van't Veer LJ, Bogaerts J, et al. 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med 2016; 375:717-729.
3. van't Veer LJ, Dai H, Van De Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature 2002; 415:530-536.
4. Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004; 351:2817-2826.
5. Sparano JA, Gray RJ, Makower DF, et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med 2015; 373:2005-2014.
6. Hattangadi J, Park C, Rembert J, et al. Breast stromal enhancement on MRI is associated with response to neoadjuvant chemotherapy. Am J Roentgenol 2008; 190:1630-1636.
7. Jones EF, Sinha SP, Newitt DC, et al. MRI Enhancement in Stromal Tissue Surrounding Breast Tumors: Association with Recurrence Free Survival following Neoadjuvant Chemotherapy. PloS one 2013; 8:e61969.
8. van der Velden BH, Dmitriev I, Loo CE, Pijnappel RM, Gilhuijs KG. Association between parenchymal enhancement of the contralateral breast in dynamic contrast-enhanced MR imaging and outcome of patients with unilateral invasive breast cancer. Radiology 2015; 276:675-685.
9. Dmitriev ID, Loo CE, Vogel WV, Pengel KE, Gilhuijs KGA. Fully automated deformable registration of breast DCE-MRI and PET/CT. Phys Med Biol 2013; 58:1221-1233.
10. Mammacarcinoom 2.0. http://www.oncoline.nl/mammacarcinoom. Accessed 18 Aug 2016. 2016.
Figures