0942
Saturated fatty acid fraction in breast adipose tissue is higher in patients with cancer than in those with benign lesions1Center for Advanced Imaging Innovation and Research, Radiology, New York University School of Medicine, New York, NY, United States
Synopsis
Gradient-echo Spectroscopic Imaging (GSI) was used to conduct voxel-wise analysis of fatty acid composition in breast adipose tissue in postmenopausal women. Parametric maps of fatty acid fractions show significantly higher saturated fatty acids in women with malignant tumors than in those with benign lesions. This result is consistent with a previous study based on manually selected regions of interest, and suggests that, for post-menopausal women, higher saturated fatty acids may be related to breast cancer development. Non-invasive evaluation of lipid composition using GSI may aid in breast cancer risk assessment and provide insight into physiological mechanisms that facilitate cancer development.
PURPOSE
In postmenopausal women, body mass index (BMI) is an important risk factor for breast cancer development and invasiveness.1 The association is due, at least in part, to the aromatization of androgens into estrogens in adipose tissue, which is the principal source of estrogens after menopause.2 Recent studies also demonstrated that lipid composition distant from lesions is different depending on the malignancy of the lesion.3 While MR-visible lipids in the tumor microenvironment have been studied extensively, the relationship between lipid composition in breast adipose tissue and cancer development remains poorly understood. One of the challenges in investigating lipid composition of the breast in the clinical setting is the lack of rapid data acquisition methods that can cover a large portion of the breast with adequate spatial resolution for the complex structure of fibroglandular and adipose tissue. Recently, Gradient-echo Spectroscopic Imaging (GSI) has been introduced to measure fractions of different fatty acids in the breast adipose tissue. It can be easily conducted during diagnostic breast MRI exams with an extra 5 minutes of acquisition time.4,5 The GSI method showed that postmenopausal women with breast cancer have a significantly higher proportion of saturated fatty acid (SFA) in breast adipose tissue than those with benign diseases.4,5 However, the analysis was conducted based on the regions of interest (ROI) manually selected by two readers. The purpose of this study was to evaluate SFA levels in an independent cohort of postmenopausal women with and without malignant lesions. Further, we assessed voxel-based analysis of fatty acid composition without interaction with a reader so that this tool may be incorporated into the clinical workflow.METHODS
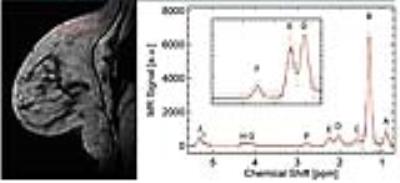
Unilateral 3D MR images with voxel-by-voxel spectroscopic information were acquired at 3T with IRB approval in 23 postmenopausal women undergoing diagnostic MRI between October 2015 and November 2015. They included 14 women without any malignant lesion (age 61.6±9.1) and 9 women with pathology-proven malignancies (age 58.1±8.8; 7 invasive ductal carcinomas, 1 invasive lobular carcinomas, and 1 ductal carcinoma in-situ). A 3D gradient echo sequence was used with the following imaging parameters: number of echoes = 144, echo spacing = 1.44 ms, TR = 220 ms, FA = 10 deg, in-plane resolution = 1.4x1.4 mm2 with zero-padding, slice thickness = 2.8 mm; number of slices = 18, scan time = ~5 min. Voxel-wise spectral analysis of fatty acids was conducted by performing a Fourier transform of the complex-valued gradient-echo image data along the echo dimension and applying an eddy current correction and zero and first-order phase corrections, followed by fitting 10 Voigt line profiles and a linear baseline. Representative final spectral fits are shown in Figure 1. Among the 10 peaks, we are interested in three resonances corresponding to allylic CH2 protons, α- to a double bond, at 2.03 ppm (D), CH2 methylene protons, α- to the carbonyl, at 2.25 ppm (E), and diallylic CH2 protons at 2.77 ppm (F). These peaks and their combinations can be used to measure relative levels of monounsaturated fatty acids (MUFA), polyunsaturated fatty acids (PUFA), and saturated fatty acids (SFA).6,7 Briefly, PUFA = area(F)/area(E); MUFA = 0.5 area(D)/area(E)-PUFA; SFA=1-PUFA-MUFA.RESULTS
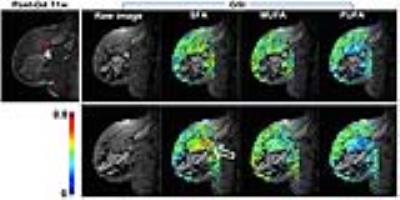
Figure 2 shows representative pixel maps of fatty acid fractions, which demonstrate higher SFA fractions near the invasive ductal carcinoma. In contrast, Figure 3 shows examples of women with benign post-surgical change or no suspicious lesion, whose SFA fractions are homogeneous across the breast and lower than that of a malignant case shown in Figure 2. In terms of mean SFA across voxels in adipose tissue (Figure 4a), post-menopausal women with malignant lesions had significantly higher SFAs (p<0.05) than those with benign tissue (0.32±0.05 vs. 0.27±0.05). Significant differences (p<0.05) were also found when comparing the minimum, median and 75th percentile of SFA (Figure 4b) distributions between groups.DISCUSSION & CONCLUSION
Inflammation in white adipose tissue triggered by SFA8,9 has been implicated in activation of estrogen production in adipocytes10,11 and also linked to development of breast cancer12-14 as well as poor prognosis15. The hormonal level in breast adipose tissue may directly affect the fibroglandular tissue in which breast adipose tissue is embedded, as substantiated by recent studies showing a majority of breast cancers are found at the interface between breast adipose tissue and fibroglandular tissue.16,17 Rapid, non-invasive evaluation of lipid composition using GSI may aid in breast cancer risk assessment, particularly for post-menopausal women, and provide insight into physiological mechanisms that facilitate cancer development.Acknowledgements
NIH R01 CA160620References
1. Research WCRFaAIfC. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington DC: AICR, 2007.
2. Nieman KM, Romero IL, Van Houten B, Lengyel E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochimica et biophysica acta. 2013;1831(10):1533-41. doi: 10.1016/j.bbalip.2013.02.010. PubMed PMID: 23500888; PubMed Central PMCID: PMC3742583.
3. He Q, Shkarin P, Hooley RJ, Lannin DR, Weinreb JC, Bossuyt VI. In vivo MR spectroscopic imaging of polyunsaturated fatty acids (PUFA) in healthy and cancerous breast tissues by selective multiple-quantum coherence transfer (Sel-MQC): a preliminary study. Magn Reson Med. 2007;58(6):1079-85. doi: 10.1002/mrm.21335. PubMed PMID: 17969083.
4. Freed M, Storey P, Lewin AA, Babb J, Moccaldi M, Moy L, Kim SG. Evaluation of Breast Lipid Composition in Patients with Benign Tissue and Cancer by Using Multiple Gradient-Echo MR Imaging. Radiology. 2016:151959. doi: 10.1148/radiol.2016151959. PubMed PMID: 27266558.
5. Freed M, Storey P, Lewin AA, Babb JS, Moccaldi M, Moy L, Kim SG, editors. Evaluation of lipid composition in patients with benign and cancer using multiple gradient echo MRI. Proc Intl Soc Mag Reson Med 23; 2015; Toronto, Canada.
6. Dimitrov IE, Douglas D, Ren J, Smith NB, Webb AG, Sherry AD, Malloy CR. In vivo determination of human breast fat composition by (1)H magnetic resonance spectroscopy at 7 T. Magn Reson Med. 2012;67(1):20-6. doi: 10.1002/mrm.22993. PubMed PMID: 21656551; PubMed Central PMCID: PMC3245342.
7. Ren J, Dimitrov I, Sherry AD, Malloy CR. Composition of adipose tissue and marrow fat in humans by 1H NMR at 7 Tesla. Journal of lipid research. 2008;49(9):2055-62. doi: 10.1194/jlr.D800010-JLR200. PubMed PMID: 18509197; PubMed Central PMCID: PMC2515528.
8. Schilling JD, Machkovech HM, He L, Sidhu R, Fujiwara H, Weber K, Ory DS, Schaffer JE. Palmitate and lipopolysaccharide trigger synergistic ceramide production in primary macrophages. The Journal of biological chemistry. 2013;288(5):2923-32. doi: 10.1074/jbc.M112.419978. PubMed PMID: 23250746; PubMed Central PMCID: PMC3561515.
9. Weber K, Schilling JD. Lysosomes integrate metabolic-inflammatory cross-talk in primary macrophage inflammasome activation. The Journal of biological chemistry. 2014;289(13):9158-71. doi: 10.1074/jbc.M113.531202. PubMed PMID: 24532802; PubMed Central PMCID: PMC3979407.
10. Iyengar NM, Hudis CA, Dannenberg AJ. Obesity and cancer: local and systemic mechanisms. Annual review of medicine. 2015;66:297-309. doi: 10.1146/annurev-med-050913-022228. PubMed PMID: 25587653.
11. Howe LR, Subbaramaiah K, Hudis CA, Dannenberg AJ. Molecular pathways: adipose inflammation as a mediator of obesity-associated cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19(22):6074-83. doi: 10.1158/1078-0432.CCR-12-2603. PubMed PMID: 23958744; PubMed Central PMCID: PMC3891839.
12. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860-7. doi: 10.1038/nature01322. PubMed PMID: 12490959; PubMed Central PMCID: PMC2803035.
13. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436-44. doi: 10.1038/nature07205. PubMed PMID: 18650914.
14. D'Alfonso TM, Ginter PS, Shin SJ. A Review of Inflammatory Processes of the Breast with a Focus on Diagnosis in Core Biopsy Samples. Journal of pathology and translational medicine. 2015;49(4):279-87. doi: 10.4132/jptm.2015.06.11. PubMed PMID: 26095437; PubMed Central PMCID: PMC4508565.
15. Iyengar NM, Zhou XK, Gucalp A, Morris PG, Howe LR, Giri DD, Morrow M, Wang H, Pollak M, Jones LW, Hudis CA, Dannenberg AJ. Systemic Correlates of White Adipose Tissue Inflammation in Early-Stage Breast Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016;22(9):2283-9. doi: 10.1158/1078-0432.CCR-15-2239. PubMed PMID: 26712688; PubMed Central PMCID: PMC4854755.
16. Yamaguchi J, Ohtani H, Nakamura K, Shimokawa I, Kanematsu T. Prognostic impact of marginal adipose tissue invasion in ductal carcinoma of the breast. American journal of clinical pathology. 2008;130(3):382-8. doi: 10.1309/MX6KKA1UNJ1YG8VN. PubMed PMID: 18701411.
17. Zhu W, Harvey S, Macura KJ, Euhus DM, Artemov D. Invasive Breast Cancer Preferably and Predominantly Occurs at the Interface Between Fibroglandular and Adipose Tissue. Clinical breast cancer. 2016. doi: 10.1016/j.clbc.2016.07.009. PubMed PMID: 27568102.
Figures