0935
Motion corrected Magnetic Resonance Fingerprinting using Soft-weighted key-Hole (MRF-McSOHO)1Division of Imaging Sciences and Biomedical Engineering, King's College London, London, United Kingdom
Synopsis
Magnetic Resonance Fingerprinting (MRF) estimates multi-parametric maps from a large amount of highly undersampled time-point images. MRF has been shown to be robust to the presence of abrupt motion occurring towards the end of the acquisition. In this work we further study the effects of different types of motion on MRF, showing high sensitivity to periodic motion and motion occurring at the beginning of the MRF scan. A method for 2D translational motion correction in MRF is proposed and validated in vivo, showing significant improvements when compared with no motion correction.
Introduction
Magnetic Resonance Fingerprinting (MRF)1 produces multi-parametric maps from a large series of highly undersampled time-point images, via template matching of a measured signal to a set of simulated signals. The original work in1 demonstrated that MRF still produces accurate parametric maps when a fraction of the time-point images is corrupted by motion. Here, we study the effects of different types of motion during the MRF acquisition and propose a novel approach to correct for this motion (MRF-McSOHO). This method is based on SOft-weighted key-HOle (MRF-SOHO2) reconstruction for highly undersampled MRF data which produces alias free time-point images, enabling motion estimation3 from the reconstructed images. Estimated motion is applied to the motion corrupted k-space, followed by a second MRF-SOHO reconstruction. The proposed approach was validated on standardized T1/T2 phantom and in-vivo brain acquisitions.Methods
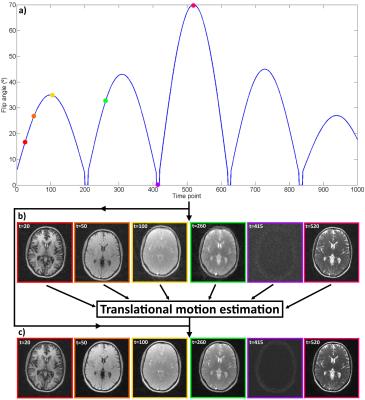
MRF-SOHO combines ideas from parallel imaging4, soft-gating5 and key-hole6 to reconstruct highly undersampled MRF data. The slow varying flip angle in the MRF acquisition produces smooth contrast changes in the time-point images, enabling high frequency data sharing in the temporal dimension. In MRF-SOHO each highly undersampled time-point image It is reconstructed by solving It = arg min ||Wtn(FSIt - Kt)||2. F is the Fourier transform, S are the coil sensitivities, Kt are the acquired k-space data for time point t and Wtn are the soft-weights for time frame t and neighbour n which control the amount of shared data between time-points. Translational motion for each time-point t is extracted from It using image registration. The corresponding phase shifts are applied to produce the motion corrected k-space K't. Finally, a second MRF-SOHO reconstruction is performed: I't = arg min ||W'tn(FSI't - K't)||2, producing the motion corrected MRF time series (Figure 1). The MRF dictionary was built with an Extended Phase Graph method based on7, using T1 ranges of [0ms, 6000ms] and [0ms, 1600ms] and T2 ranges of [0ms, 2600ms] and [0ms, 260ms] for brain and phantom, respectively. Template matching was performed as described in1.Experiments
A standardized T1/T2 phantom6 was scanned in a 1.5T Philips MR scanner using an 18-channel coil. Data was obtained with a rewound gradient echo acquisition after an initial inversion pulse using a tiny golden radial (~23.6º) trajectory7. Relevant scan parameters include: resolution 2x2 mm2, 10 mm slice thickness, field-of-view 320x320 mm2, 8 radial spokes per time-point, 1000 time points and TR (varying between 6.0 and 7.4 ms) and FA (varying between 0º and 70º) patterns similar to the ones described in8. One healthy subject underwent a brain scan using a 12-channel coil and the same protocol as above (except field-of-view=440x440 mm2). Three motion experiments were performed by modifying the acquired motion free k-space: 1) abrupt 2D translational motion in the last 100 time-points, 2) abrupt 2D translational motion in the first 100 time-points, 3) realistic 2D respiratory motion throughout all time-points based on previously acquired respiratory signal. The MRF-SOHO neighbourhood size was set to 11 and 21 for the reconstructions before and after motion correction, respectively. All motion experiments were reconstructed with a zero fill gridding reconstruction as in conventional MRF1 with and without motion, and with the proposed MRF-McSOHO (motion corrected).Results
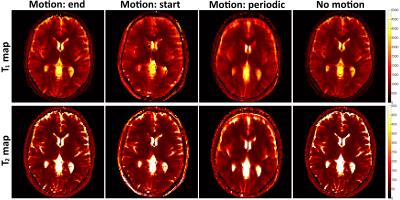
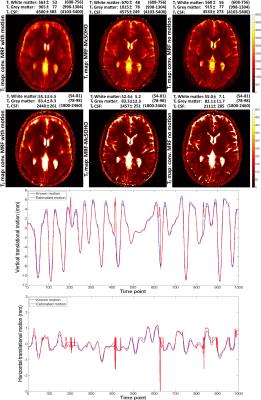
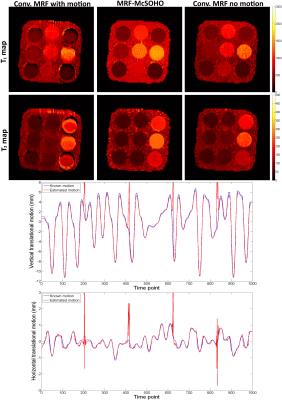
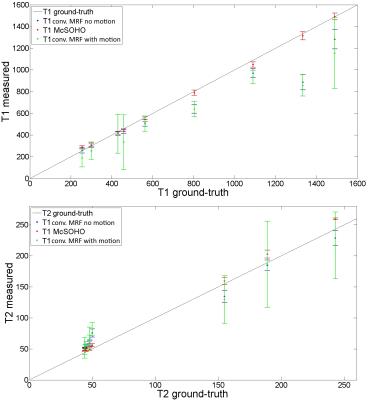
Brain T1 and T2 maps for the different type of motions studied are shown in Figure 2 for conventional MRF, demonstrating MRF is robust to motion in the end (as shown in1), but sensitive to other types of motion. Brain T1 and T2 maps and motion estimation plots (in comparison with ground truth) are shown in Figure 3 for conventional MRF and the proposed MRF-McSOHO. Corresponding maps and plots for the phantom dataset can be seen in Figure 4. In both cases artefacts can be seen in conventional MRF in the presence of motion, however these are significantly reduced with the proposed method. 2D reliable motion estimation was obtained for most time-points, except when the flip angle was close to zero. Regardless, no significant artefacts were found in the time-point images or the parametric maps (Figures 3 and 4). T1 and T2 measurements of the standardized phantom in comparison to ground-truth values are shown in Figure 5, where MRF-McSOHO achieves higher accuracy and precision than conventional MRF (with and without motion). Additionally, underestimation of high T1 values was observed for conventional MRF in both datasets, however the proposed method correctly estimated these values.Conclusion
MRF was shown to be sensitive to periodic motion. A novel method for motion correction in MRF (MRF-McSOHO) was proposed, improving parametric map quality and accuracy. Future work will include further validations in-vivo for different types of motion and different applications.Acknowledgements
ACKNOWLEGDMENTS: This work was supported by EPSRC EP/P001009/1 and FONDECYT 1161055.References
1. Ma D et al. Magnetic Resonance Fingerprinting. Nature. 2013; 495:187-192
2. Cruz et al. Accelerated Magnetic Resonance Fingerprinting using Soft-weighted key-Hole (MRF-SOHO). ISMRM. 2017. Submitted
3. Buerger et al. Hierarchical adaptive local affine registration for fast and robust respiratorymotion estimation. Med Image Anal. 2011; 15:551-564
4. Pruessman et al. Advances in sensitivity encoding with arbitrary k-space trajectories. MRM. 2001; 46:638-651
5. Johnson et al. Improved Least Squares MR Image Reconstruction Using Estimates of k-Space Data Consistency. MRM. 2012; 67:1600-1608
6. Song et al. Non-Contrast Enhanced 4-D Dynamic MRA with Golden Angle Radial Acquisition and K-space Weighted Image Contrast (KWIC) Reconstruction. MRM. 2004; 52:815-824
7. Weigel. Extended Phase Graphs: Dephasing, RF Pulses,and Echoes - Pure and Simple. MRM. 2015; 41:266-295
8. Jiang et al. MR Fingerprinting Using Fast Imaging with Steady State Precession (FISP) with Spiral Readout. MRM. 2015; 74:1621-1631
Figures