0929
Characterisation of in-vivo and ex-vivo cardiac Diffusion Tensor Imaging scalar measures of cardiac microstructure in healthy swine1NHLBI, NIH, Bethesda, MD, United States, 2Royal Brompton Hospital, Imperial College of London, London, United Kingdom, 3Department of Radiological Sciences, University of California Los Angeles, Los Angeles, CA, United States
Synopsis
In-vivo cDTI was performed at several cardiac phases in healthy swine (N=16), followed by ex-vivo cDTI in two contractile states. The three eigenvalues (L1, L2, L3), MD, FA and Mode were compared. All trends between in-vivo diastole and systole matched those between ex-vivo relaxed and contracted states, except for MD, which decreased ~10% in-vivo from diastole to systole, with no significant differences ex-vivo between relaxed and contracted states. These results provide a useful baseline for future preclinical studies with cardiac disease models, and might contribute towards formulating a strain correction model that accounts for the microstructural constraints and deformations of the myocardium.
Background
In-vivo cDTI monopolar STEAM acquisition techniques have been previously reported, providing cDTI measures of microstructure in the normal human heart (1-4), and in patients with hypertrophic cardiomyopathy (5), dilated cardiomyopathy (6) and myocardial infarct (7). Because of the potential influence of cyclical strain on in-vivo cDTI STEAM measures (8,9) uncertainty has remained regarding the interpretation of in-vivo cDTI STEAM data published.Objectives
To characterise in-vivo cDTI STEAM scalar measures of cardiac microstructure throughout the cardiac cycle and compare them to ex-vivo values in both relaxed and contracted states in healthy swine.Methods
Animal procedures were approved by the National Heart, Lung, and Blood Institute Animal Care and Use Committee. In-vivo cDTI was performed in Yorkshire pigs (N=16) at two mid-ventricular short-axis slices and at 6-9 time points during the cardiac cycle. Subsequently, cardiac arrest was induced in both relaxed (N=8) and contracted (N=8) states by intravenous administration of potassium chloride (KCl) (10) and barium chloride (BaCl2) (10), respectively. All hearts were excised and imaged by ex-vivo cDTI. The in-vivo breath-hold and ex-vivo cDTI STEAM EPI sequence with monopolar diffusion encoding is described elsewhere (1,6): b0=50s/mm2 (20 averages), b=500s/mm2 in 6 diffusion encoding directions (20 averages), as well as b=150s/mm2, b=350s/mm2 in 6 directions (2 averages), FOV=320mm×120mm, spatial resolution = 2x2x8mm3 interpolated to 1x1x8mm3. All the diffusion-weighted images were post processed with custom Matlab software (Mathworks) (5). The three eigenvalues (L1, L2, and L3), as well as mean diffusivity (MD), fractional anisotropy (FA) and tensor mode (11,12) were analysed. Changes in cDTI scalar values between diastole and systole in-vivo were assessed with a Wilcoxon signed rank test. Changes in cDTI scalar values between relaxed and contracted states ex-vivo were assessed with a Mann–Whitney–Wilcoxon (MWW) test using MedCalc Statistical Software. A p-value < 0.01 was considered significant.Results
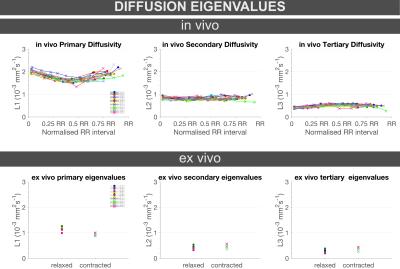
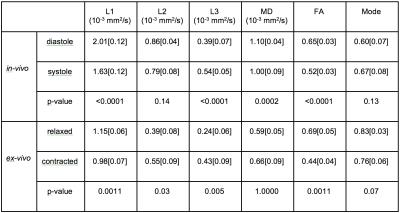
L1 significantly decreased both in-vivo from diastole (median[interquarile range], 2.01[0.12]×10-3mm2/s) to systole (1.63[0.12]×10-3mm2/s, p<0.0001), and ex-vivo from relaxed (1.15[0.06]×10-3mm2/s) to contracted (1.98[0.07]×10-3mm2/s, p=0.0011) states (Figure 1, Table 1).
L2 showed no significant changes either in-vivo from diastole (0.86[0.04]×10-3mm2/s) to systole (0.79[0.08]×10-3mm2/s, p=0.14), or ex-vivo between relaxed (0.39[0.08]×10-3mm2/s) and contracted (0.55[0.09]×10-3mm2/s, p=0.03) states (Figure 1, Table 1).
L3 significantly increased both in-vivo from diastole (0.39[0.07]×10-3mm2/s) to systole (0.54[0.05]×10-3mm2/s, p<0.0001), and ex-vivo from relaxed (0.24[0.06]×10-3mm2/s) to contracted (0.43[0.09]×10-3mm2/s, p=0.005) states (Figure 1, Table 1).
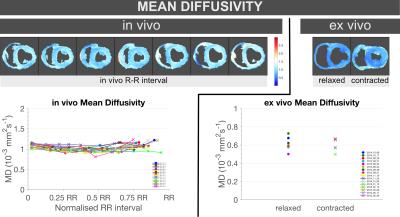
MD significantly decreased by ~10% in-vivo from diastole (1.10[0.04]×10-3mm2/s) to systole (1.00[0.09]×10-3mm2/s, p=0.0002), while no significant differences were found ex-vivo between relaxed (0.59[0.05]×10-3mm2/s) and contracted (0.66[0.09]× 10-3mm2/s, p=1.000) states (Figure 2, Table 1).
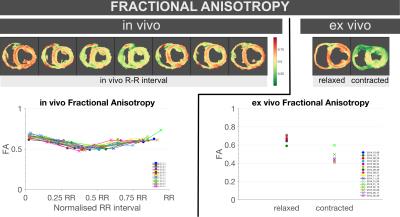
FA significantly decreased both in-vivo between diastole (0.65[0.03]) and systole (0.52[0.03], p<0.0001), and ex-vivo between relaxed (0.69[0.05]) and contracted (0.44[0.04], p=0.0011) states (Figure 3, Table 1).
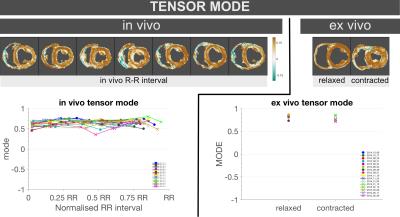
No significant differences in Mode were found either in-vivo from diastole (0.60[0.07]) to systole (0.67[0.08], p=0.13), or ex-vivo between relaxed (0.83[0.03]) and contracted (0.76[0.06], p=0.07) states (Figure 4, Table 1).
Discussion and Conclusion
This study provides scalar cDTI values at multiple phases throughout the cardiac cycle in-vivo in healthy swine, as well as ex-vivo in relaxed and contracted states. These results provide a useful baseline for future preclinical studies with cardiac disease models.
Eigenvalues and MD were significantly lower ex-vivo compared to in-vivo. Changes in temperature, microcirculation and tissue vitality, as well as SNR changes between in-vivo and ex-vivo hearts may account for these differences.
All trends between in-vivo diastole and systole matched those between ex-vivo relaxed and contracted states, except MD, which decreased ~10% in-vivo from diastole to systole, with no significant differences ex-vivo between relaxed and contracted states.
The decrease in FA from relaxed to contracted states is consistent with the shortening and thickening associated with systolic contraction. The similar values of mode between in-vivo and ex-vivo data despite changes in FA suggest that the tensor maintains its relative shape.
This data might help contribute towards formulating a strain correction model (8,9) that takes into account the microstructural constraints and deformations of the myocardium (5,13).
Acknowledgements
The authors would like to thank Joni Taylor, Shawn Kozlov, Katherine Lucas for expert animal care and Rick Wage and Gill Smith for their expert CMR technical help. We would also like to thank Prof. Hewitt, Dr. Candice Perry and Dr. Kris Ylaya for the use of the Nanozoomer. This work was supported by the following:
- National Heart, Lung and Blood Institute, National Institutes of Health by the Division of Intramural Research, NHLBI, NIH, DHHS (HL00460714CPB).
- British Heart Foundation.
- National Institute of Health Research Cardiovascular Biomedical Research Unit at the Royal Brompton Hospital and Imperial College, London.
References
1. Nielles-Vallespin S, Mekkaoui C, Gatehouse P et al. In vivo diffusion tensor MRI of the human heart: reproducibility of breath-hold and navigator-based approaches. Magn Reson Med 2012;70:454-65.
2. Scott AD, Ferreira PF, Nielles-Vallespin S et al. Optimal diffusion weighting for in vivo cardiac diffusion tensor imaging. Magn Reson Med 2015;74:420-30.
3. Edelman R, Gaa J, Wedeen V et al. In-vivo measurement of water diffusion in the human heart. Magn Reson Med 1994;32:423-8.
4. Reese TG, Weisskoff RM, Smith RN, Rosen BR, Dinsmore RE, Wedeen VJ. Imaging myocardial fiber architecture in vivo with magnetic resonance. Magn Reson Med 1995;34:786-91.
5. Ferreira PF K, McGill L, Nielles-Vallespin S et al. In vivo cardiovascular magnetic resonance diffusion tensor imaging shows evidence of abnormal myocardial laminar orientations and mobillity in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson 2014;16:87.
6. Sonia Nielles-Vallespin ZK, Pedro F. Ferreira, Ranil de Silva, Andrew D. Scott, Philip Kilner, Laura-Ann McGill, Archontis Giannakidis, Peter D. Gatehouse, Daniel Ennis, Eric Aliotta, Majid Al-Khalil, Peter Kellman, Dumitru Mazilu, Robert S. Balaban, David N. Firmin, Andrew E. Arai, Dudley J. Pennell. Myocardial microstructural dynamics by in-vivo diffusion tensor CMR: preclinical validation and clinical translation in HCM and DCM. JACC 2016.
7. Wu E, Wu Y, Nicholls J, Wang J, Liao S, Zhu S. MR diffusion tensor imaging study of postinfarct myocardium structural remodeling in a porcine model. Magn Reson Med 2007;58:687 - 695.
8. Reese T, Wedeen V, Weisskoff R. Measuring diffusion in the presence of material strain. J Magn Reson B 1996;112:253-8.
9. Tseng W, Reese T, Weisskoff R, Wedeen V. Cardiac diffusion tensor MRI in vivo without strain correction. Magn Reson Med 1999;42:393 - 403.
10. Chen J, Liu W, Zhang H et al. Regional ventricular wall thickening reflects changes in cardiac fiber and sheet structure during contraction: quantification with diffusion tensor MRI. Am J Physiol Heart Circ Physiol 2005;289:H1898 - 907.
11. Ennis DB, Kindlman G, Rodriguez I, Helm PA, McVeigh ER. Visualization of tensor fields using superquadric glyphs. Magn Reson Med 2005;53:169-76.
12. Ennis DB, Kindlmann G. Orthogonal tensor invariants and the analysis of diffusion tensor magnetic resonance images. Magn Reson Med 2006;55:136-46.
13. Axel L, Wedeen V, Ennis D. Probing dynamic myocardial microstructure with cardiac magnetic resonance diffusion tensor imaging. Journal of Cardiovascular Magnetic Resonance 2014;16:89.
Figures