0927
In Vivo Assessment of Cardiomyocyte Performance Using Combined Cardiac DENSE and cDTI1Department of Bioengineering, University of California, Los Angeles, CA, United States, 2Department of Radiological Sciences, University of California, Los Angeles, CA, United States, 3Biomedical Physics IDP, University of California, Los Angeles, CA, United States
Synopsis
Circumferential strain (Ecc) derived from cardiac DENSE 3D displacement maps is a promising biomarker for diagnosing early stages of cardiac disease. Ecc is commonly evaluated only in the mid-wall where it is expected to align with the local myofiber direction. Our aim was to combine cDTI and DENSE displacement data to directly characterize strain along the fiber direction (Eff) throughout the heart. Across all healthy subjects (N=9), Eff had a smaller peak systolic transmural gradient than Ecc (-0.035±0.041 vs. -0.097±0.030 (unitless), p<0.001). Eff is a more spatially uniform measure of regional LV function in healthy volunteers and provides a microstructurally anchored measure of cardiomyocyte performance.
Background
Quantitative MRI biomarkers of regional myocardial performance may outperform global measurements of function (e.g. ejection fraction, EF) in diagnosing the earliest signs of cardiac disease and response to therapy. Displacement Encoding with Stimulated Echoes (DENSE) has shown particular sensitivity to changes in circumferential strain (Ecc) in, for example, acute MI and hypertrophic cardiomyopathy 1,2. Current measures of strain, however, are not inherently linked to the underlying left ventricular (LV) microstructure, which cannot be inferred by the direction of the principal strains. Consequently, current strain measures are unable to directly characterize myocyte performance.
LV mid-wall Ecc has a steep transmural gradient due to a lack of alignment with cardiomyocyte orientation outside of the mid-wall which limits the precision and repeatability of Ecc measurements. Assessing strain along the local myofiber direction (Eff) would provide a microstructurally anchored measure of cardiomyocyte performance throughout the myocardium, but also requires additional microstructural imaging.
Leveraging recent advances in cardiac diffusion tensor imaging (cDTI) that enable direct measurement of in vivo cardiomyocyte orientations 4,5, our objectives were: 1) to combine cDTI and DENSE displacement data to measure Eff in healthy subjects; 2) to demonstrate that cDTI myofiber orientation provides information not contained in the strain tensor; and 3) to compare the transmural variability of Eff and Ecc.
Methods
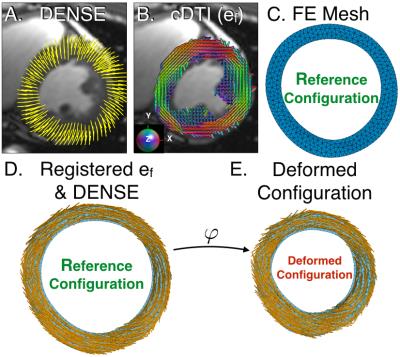
Imaging: A single mid-ventricular short-axis slice was acquired with cDTI and DENSE in healthy volunteers (N=9, 2 females, 7 males, aged 25-42) at 3T (Siemens Prisma) after obtaining informed consent. Imaging parameters were: cDTI (mid-systolic phase): 2x2x5mm, TE/TR=65/~3000ms, b-value=0,350s/mm2, Navg=5, Ndir=12, free breathing with respiratiory triggering, scan time=4min; DENSE: balanced 4-point encoding, 2.5x2.5x8mm, TE/TR=1.04/15, Ke=0.06 cycles/mm, Navg=3, spiral interleaves=10, scan time=5min. Both used navigator gating.
Post-processing: Lagrangian displacements were first extracted from DENSE data with custom software 6. cDTI myofiber vectors were reconstructed and registered with the DENSE data and mapped onto a linear, triangular finite element (FE) mesh of the acquired short-axis slice. FE mesh interpolation was used to compute the continuous displacement field, the deformation gradient tensor F, and the strain E=1/2(FTF-I) from the end-diastolic reference configuration. The projections of E along the myofiber and local circumferential directions correspond, respectively, to Eff and Ecc. The eigenvectors of E provided the principal strain directions (e1, e2, and e3) and values (E1-E3). Across all volunteers, the angular differences (Δθ, mean±SD) between fiber (ef ), circumferential, (ec), and e1 to e3 directions were measured, and the values of Eff, Ecc, and E1 to E3 were compared using two-sided, paired t-tests.
Throughout the LV myocardium, peak systolic Eff and Ecc for each volunteer were binned by percent wall depth in 20% increments from epicardium (0%) to endocardium (100%). Each bin was averaged across subjects and the transmural gradients of Eff and Ecc were estimated using linear regression and compared using a two-sided, paired t-test.
Results
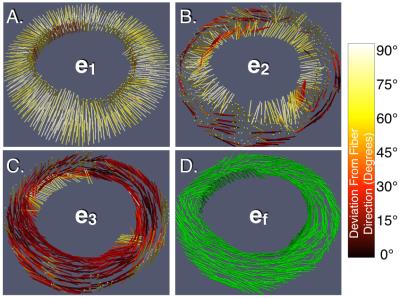
Figure 2 shows mid-systolic ef derived from cDTI and angular differences between these fiber orientations and e1, e2, e3, and ec derived from DENSE. Across all locations in all volunteers, there was poor angular agreement between ef and e1 (Δθ=77.1±11.4°) and e2 (Δθ=59.9±22.5°) and moderate agreement with e3 (Δθ=37.1±23.5°) and ec (Δθ=40.0±23.5°). Across all volunteers, significant differences were observed between E1, E2, and E3 and Eff or Ecc, Particularly, peak-systolic E3, Ecc, and Eff were significantly different (-21.1±4.9%, -16.8±6.0%, and -11.3±11.3%, p<0.001). Globally, Eff was 27% lower than mid-wall Ecc.
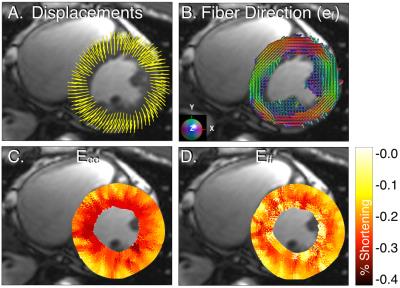
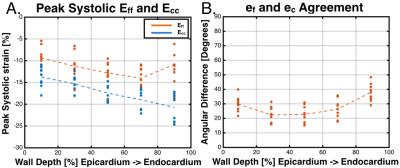
Figure 3 shows DENSE displacements, ef, and peak Eff and Ecc for a single healthy volunteer. Across all volunteers, Ecc had a larger peak systolic transmural gradient (%-strain per %-wall depth) than Eff (-0.097±0.030 vs. -0.035±0.041, p<0.001) (Figure 4A). Figure 4B shows the agreement between the local fiber and circumferential directions binned by percent of wall depth. Agreement is highest at mid-wall (Δθ=22.3±5.9°) and lower at the endocardium (Δθ=37.9±6.5°) and epicardium (Δθ=29.8.±5.7°).
Discussion
Here we report a novel combination of cDTI and cine DENSE using a continuum mechanics framework to directly measure Eff. Unlike principal or circumferential strain, this measure incorporates microstructural information that cannot be ascertained using DENSE data alone. Dou et al. suggest that myofiber shortening contributes to circumferential strain, but is not corrupted by elements of wall thickening 7. Our results indicate that Eff < Ecc, suggesting that other strain components contribute to Ecc or that ec and ef are not coaxial even at the mid-wall, which we observed. The decreased transmural gradient in Eff suggests that it may serve as a less geometry-dependent measure of regional function.Conclusion
Combining cardiac DENSE and cDTI enables the calculation of Eff, a microstructurally anchored measure of cardiomyocyte performance.Acknowledgements
Funding support from the Department of Radiological Sciences, NIH R01HL131975, and the Center for Duchenne Muscular Dystrophy at UCLA.References
1. Aletras AH, Tilak GS, Hsu L-Y, Arai AE. Heterogeneity of intramural function in hypertrophic cardiomyopathy mechanistic insights from MRI late gadolinium enhancement and high-resolution displacement encoding with stimulated echoes strain maps. Circulation: Cardiovascular Imaging 2011;4(4):425-434.
2. Aletras AH, Tilak GS, Natanzon A, et al. Retrospective determination of the area at risk for reperfused acute myocardial infarction with T2-weighted cardiac magnetic resonance imaging histopathological and displacement encoding with stimulated echoes (DENSE) functional validations. Circulation 2006;113(15):1865-1870.
3. Ennis DB, Nguyen TC, Riboh JC, et al. Myofiber angle distributions in the ovine left ventricle do not conform to computationally optimized predictions. Journal of biomechanics 2008;41(15):3219-3224.
4. Aliotta E, Wu HH, Ennis DB. Convex optimized diffusion encoding (CODE) gradient waveforms for minimum echo time and bulk motion–compensated diffusion-weighted MRI. Magnetic resonance in medicine 2016.
5. Tseng WYI, Wedeen VJ, Reese TG, Smith RN, Halpern EF. Diffusion tensor MRI of myocardial fibers and sheets: Correspondence with visible cut-face texture. Journal of Magnetic Resonance Imaging 2003;17(1):31-42.
6. Spottiswoode BS, Zhong X, Hess A, et al. Tracking myocardial motion from cine DENSE images using spatiotemporal phase unwrapping and temporal fitting. IEEE Transactions on medical imaging 2007;26(1):15-30.
7. Dou J, Tseng WYI, Reese TG, Wedeen VJ. Combined diffusion and strain MRI reveals structure and function of human myocardial laminar sheets in vivo. Magnetic Resonance in Medicine 2003;50(1):107-113.
Figures