0924
4D Flow MRI, Cardiac Function, and Myocardial T1-Mapping: Ventricular-arterial coupling in Patients with Bicuspid Aortic Valve (BAV)1Radiology, Northwestern University, Chicago, IL, United States, 2Biomedical Engineering, Northwestern University, Chicago, IL, United States
Synopsis
BAV is the most prevalent congenital cardiovascular malformation. Its association with progressive ascending aortic dilatation and concomitant aortic valve stenosis or regurgitation with increasing age has a critical impact on patients’ morbidity. We applied a comprehensive CMR protocol in 50 BAV patients consisting of cine-imaging, T1-mapping and 4D flow MRI to simultaneously assess cardiac parameters and aortic hemodynamics. We observed significant relationships between LV mass and WSS as well as peak velocities in the AAo and arch, likewise in the sub-cohort with normal valve function, leading us to the hypothesis that there is proof for ventricular-aortic coupling in BAV patients.
Purpose
Methods
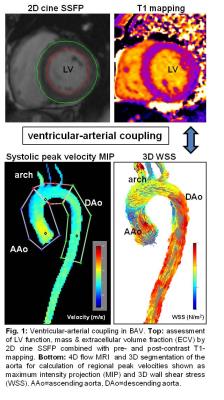
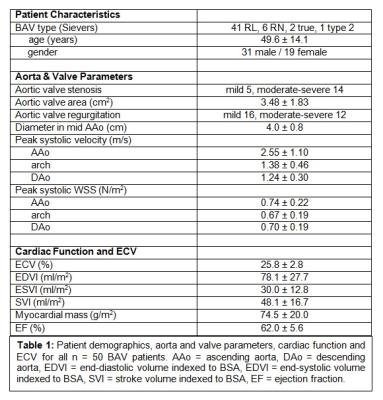
With institutional IRB approval, 50 BAV patients (age 49.6±14.1 years, table 1) were retrospectively enrolled. All patients underwent CMR including conventional SSFP-cine-imaging in the short axis orientation for the assessment of global cardiac function (end-diastolic (LVEDV)/ end-systolic volumes (LVESV), stroke volumes, ejection fraction) and myocardial mass (Syngo Argus, Siemens, Germany). Pre- and post-contrast T1-mapping was acquired (Modified Look-Locker inversion recovery sequence (MOLLI), double dose Gadobutrol (Gadavist)) for the assessment of ECV in the 16 segment AHA model in basal, mid-ventricular and apical short axis slices (Circle 5.3, Canada). Cine-SSFP and 2D phase-contrast measurements were performed at the level of the aortic valve (AV) to measure valve area, grade of AS and AR according to international guidelines 8. Cross-sectional mid AAo diameter was measured in multiplanar reformatted MRA-images. In addition, 4D flow MRI (venc 250cm/s, spatial resolution (2.2-2.8mm)3, temporal resolution 38-40ms) was acquired with full 3D coverage of the thoracic aorta. All 4D flow data were collected during free breathing using adaptive diaphragm navigator gating. 4D flow MRI data analysis included pre-processing (noise masking, phase offset error corrections, velocity anti-aliasing) and 3D segmentation of the aorta (Mimics, Materialise, Leuven, Belgium). The 3D segmentation was used to mask 4D flow velocity data for the quantification of peak systolic velocities and 3D WSS in the ascending aorta (AAo), arch, and descending aorta (DAo) (fig. 1).
Results
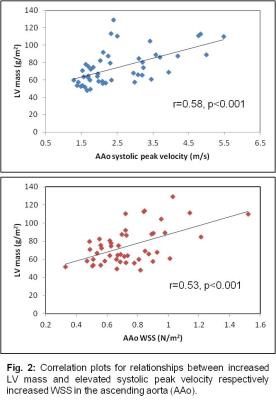
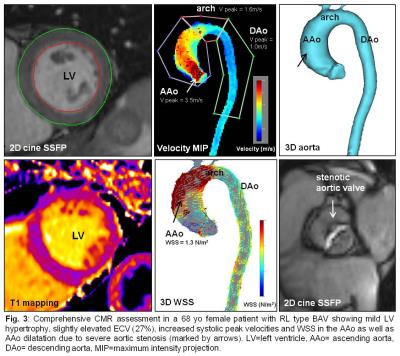
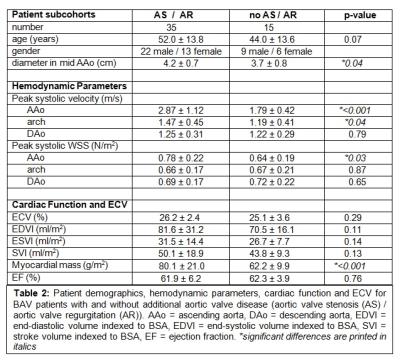
Global ECV (25.8±2.8%) and LV function (EF=62.0±5.6%) were within the normal ranges. Indexed LV mass was 74.5±20.0mg/m2. Ventricular-aortic analysis in the complete cohort revealed significant relationships between increased LV mass and elevated peak systolic velocity (r=0.58, p<0.001) and increased WSS in the AAo (r=0.53, p<0.001) (fig. 2). After subdividing the BAV cohort into patients with and without aortic valve dysfunction (AS/AR), we detected significantly increased mean systolic WSS in the AAo (p=0.03), peak systolic velocities in the AAo (p=<0.001) and arch (p=0.04), LV mass (p=<0.001), and AAo diameter (p=0.04) in the AS/AR group (table 2, fig. 3). In patients with AS/AR, there were significant associations between elevated WSS and peak velocities in the arch with increased LVEDV (r=0.64, p=<0.001; r=0.35, p=0.4) and with increased LVESV (r=0.58, p<0.001; r=0.37, p=0.03) which were not present in the other group. Finally, a significant association was found between increased AAo WSS and elevated ECV for the complete cohort (r=0.31, p=0.03) and the AS/AR group (r=0.41, p=0.01) but not for BAV patients with normal aortic valve function.Discussion
Our results show that there is evidence for ventricular-arterial coupling in BAV patients: Valve mediated increases in peak systolic velocities and WSS were associated with increased myocardial mass. Notably, this relationship was preserved even for the small group of patients without additional AS or AR. Other associations between cardiac and hemodynamic parameters in our study, including the association between ECV and AAo WSS, were strongly affected by presence of AS and AR. Thus, aortic valve dysfunction results in more severe LV remodeling which also leads to interstitial fibrosis and was not seen for BAV patients with normal valve function.Conclusion
AS and AR have a major impact on cardiac parameters and aortic hemodynamics in BAV patients. However, the relationships between hemodynamics in the AAo and arch and the myocardial mass in patients without AS and AR demonstrate that BAV patients have features of ventricular-aortic coupling.
Acknowledgements
Grant funding by NIH R01 HL115828 and K25 HL119608References
1. Siu SC, Silversides CK. Bicuspid aortic valve disease. Journal of the American College of Cardiology. 2010;55(25):2789-2800.
2. Michelena HI, Della Corte A, Prakash SK, Milewicz DM, Evangelista A, Enriquez-Sarano M. Bicuspid aortic valve aortopathy in adults: Incidence, etiology, and clinical significance. International journal of cardiology. 2015;201:400-407.
3. Allen BD, van Ooij P, Barker AJ, et al. Thoracic aorta 3D hemodynamics in pediatric and young adult patients with bicuspid aortic valve. Journal of magnetic resonance imaging : JMRI. 2015;42(4):954-963.
4. van Ooij P, Potters WV, Collins J, et al. Characterization of abnormal wall shear stress using 4D flow MRI in human bicuspid aortopathy. Annals of biomedical engineering. 2015;43(6):1385-1397.
5. Barker AJ, Markl M, Burk J, et al. Bicuspid aortic valve is associated with altered wall shear stress in the ascending aorta. Circulation Cardiovascular imaging. 2012;5(4):457-466.
6. Mahadevia R, Barker AJ, Schnell S, et al. Bicuspid aortic cusp fusion morphology alters aortic three-dimensional outflow patterns, wall shear stress, and expression of aortopathy. Circulation. 2014;129(6):673-682.
7. Guzzardi DG, Barker AJ, van Ooij P, et al. Valve-Related Hemodynamics Mediate Human Bicuspid Aortopathy: Insights From Wall Shear Stress Mapping. Journal of the American College of Cardiology. 2015;66(8):892-900.
8. American College of C, American Heart Association Task Force on Practice G, Society of Cardiovascular A, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Journal of the American College of Cardiology. 2006;48(3):e1-148.