0915
Ventral and dorsal horn grey matter neurodegeneration remote from stenosis in cervical spondylotic myelopathy1Spinal Cord Injury Center, University of Zurich, Zurich, Switzerland, 2Department of Systems Neuroscience, University Medical Center Hamburg-Eppendorf, Hamburg, Germany, 3Department of Neurophysics, Max Planck Insitute for Human Cognitive and Brain Sciences, Leipzig, Germany, 4Wellcome Trust Centre for Neuroimaging, UCL Institute of Neurology, London, United Kingdom, 5Department of Brain Repair and rehabilitation, UCL Institute of Neurology, London, United Kingdom
Synopsis
Cervical spondylotic myelopathy (CSM) is the most frequent spinal cord disorder. Next to focal degeneration at the compression site, the rostral cervical white and grey matter undergo atrophic changes, the magnitude relating to clinical impairment. In this study, we assess above stenosis regional grey matter changes and demonstrate that next to bilateral dorsal horn atrophy, the normal appearing ventral horns show diffusivity changes which relate to white matter integrity loss.
Purpose
Cervical spondylotic myelopathy (CSM) induces spinal cord degeneration and gradually leads to functional impairment and reduced quality of life.1 Next to focal myelopathy, widespread atrophy has been identified in the cervical cord grey and white matter above the level of stenosis2 and brain.3 This study investigates detailed grey matter volume and microstructural changes within the cervical dorsal and ventral horns above stenosis.Methods
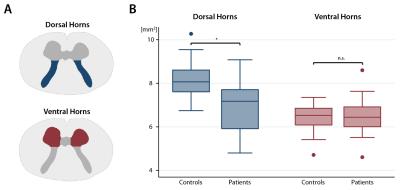
Twenty patients with CSM underwent a comprehensive clinical assessment including the modified Japanese Orthopaedic Association (mJOA) scale [max. 18 points].4 Eighteen healthy subjects were enrolled as controls. All participants were scanned on a 3T Skyrafit MRI scanner (Siemens, Germany). To investigate the cervical grey matter, we used a high-resolution T2*-weighted MEDIC sequence (3D multi-echo spoiled GRE) for volumetric changes and a diffusion weighted (DW) dataset using a cardiac-gated reduced field of view single-shot spin-echo EPI sequence with outer volume suppression5,6 (30 DW images (b=500s/mm2), 6 T2-weighted images (b=0s/mm2)) for microstructural changes above the level of compression at vertebra C2/C3. We manually segmented cross-sectional grey matter2 and subdivided the latter into the bilateral dorsal horns (approximately Rexed lamina I-V) and ventral horns (Rexed lamina VI-IX) excluding lamina X (Figure 1A). To the diffusion data, we applied eddy current and motion correction7,8 and robust tensor fitting8 to adjust diffusion data for motion, instrumental and physiological artefacts using the ACID toolbox in SPM12.9 We then spatially normalized mean diffusivity (MD) and fractional anisotropy (FA) maps to the MNI-Poly-AMU template.10 We used two-sample t-tests within STATA to assess differences in the areas of the dorsal and ventral horns between patients and controls. Moreover, voxel-wise comparison of DTI data within the grey matter atlas10 as region of interest was performed within the framework of SPM12. Regression models (voxel-wise for DTI data) were constructed to assess correlations between grey matter MRI readouts and impairment and between the integrity of grey matter and the corresponding white matter tract (e.g. ventral horn and lateral corticospinal tract (CST)). A cluster-defining threshold of p<0.01 uncorrected was initially used for all statistical parametric maps and Gaussian Random Field theory was applied to account for multiple comparisons (p<0.05).11Results
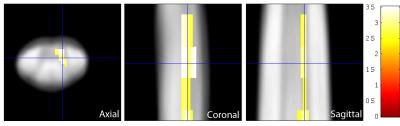
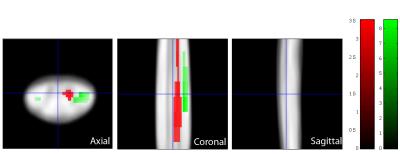
Ten patients suffered from mild (mJOA>14), nine from moderate (mJOA=12-14), and one from severe (mJOA <12) CSM with mainly sensory impairment. We first confirmed reduced cross-sectional area of grey matter by 7.1% and loss of microstructural integrity within the white matter of the lateral CST and dorsal columns in patients.2 Grey matter sub-segmentation revealed reductions by 15.6% in the cross-sectional area (mean±SD) of the dorsal horns (p=0.0004; patients: 6.98±1.18mm2; controls 8.27±0.85mm2), whereas the area of the ventral horns was not significantly different (p=0.7281; patients: 6.46±0.87mm2; controls: 6.54±0.46mm2) (Figure 1B). Coefficient of variations for grey matter, dorsal horn, and ventral horn areas were 2.8%, 3.6%, 5.6% for intra-rater and 7.0%, 11.7%, and 17.1% for inter-rater (two independent investigators) variations. As opposed to morphometry, no significant diffusivity changes were observed in the dorsal horns. Instead, MD within the right ventral horn was elevated in patients compared to controls (p=0.045, k=67, Z=3.22, x=-2.5mm, y=-19mm, z=36mm) (Figure 2). No changes were observed for FA. Testing for associations between white and grey matter diffusivities, we found that elevated MD within the right ventral horn correlated with elevated MD (p=0.013, k=98, Z=5.25, x=3.5mm, y=-19.5mm, z=19mm) (Figure 3) and reduced FA (p=0.028, k=74, Z=4.53, x=4.5mm, y=-18.5mm, z=30mm) within the corresponding right lateral CST.Discussion
Atrophy in the dorsal horns (e.g. sensory neurons) remote to the site of compression reflects the more prominent sensory impairment (especially pain). While patients had only mild motor impairment, changes in diffusivities were observed within the right ventral horn; the extent correlated with remote diffusivity changes within the lateral CST. The same finding on the contralateral side did not survive multiple comparison correction. Crucially, significant microstructural changes as well as the interplay between the extent of grey and white matter diffusivity changes indicate the effect of neurodegeneration on the motor system prior to marked clinical impairment. No relationship between clinical assessments and extent of grey matter degeneration was observed, since sensorimotor function depends on integrity of grey and white matter across the neuraxis.Conclusion
Assessing regional grey matter changes remote to the site of compression reveals focal neurodegeneration in CSM patients with mild motor impairment. Therefore these MRI readouts may serve as biomarkers that are sensitive to subclinical structural changes and thereby might supplement clinical readouts.Acknowledgements
This work was supported by the Clinical Research Priority Program “Neuro-Rehab” of the University of Zurich and the International Foundation for Research in Paraplegia.References
1. Kalsi-Ryan, S., Karadimas, S. K. & Fehlings, M. G. Cervical spondylotic myelopathy: the clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist 19, 409–421 (2013).
2. Grabher, P. et al. Voxel-based analysis of grey and white matter degeneration in cervical spondylotic myelopathy. Sci. Rep. 6, 24636 (2016).
3. Aleksanderek, I. et al. Cervical Spondylotic Myelopathy: Metabolite Changes in the Primary Motor Cortex after Surgery. Radiology 000, 152083 (2016).
4. Benzel, E. C., Lancon, J., Kesterson, L. & Hadden, T. Cervical laminectomy and dentate ligament section for cervical spondylotic myelopathy. Journal of spinal disorders 4, 286–295 (1991).
5. Morelli, J. N. et al. Evaluation of a modified Stejskal-Tanner diffusion encoding scheme, permitting a marked reduction in TE, in diffusion-weighted imaging of stroke patients at 3 T. Invest. Radiol. 45, 29–35 (2010).
6. Heidemann, R. M. et al. High resolution single-shot diffusion-weighted imaging with a combination of zoomed EPI and parallel imaging. in Proceedings of the 17th Annual Meeting of ISMRM, Honolulu, USA, p. 2736 (2009).
7. Mohammadi, S., Möller, H. E., Kugel, H., Müller, D. K. & Deppe, M. Correcting eddy current and motion effects by affine whole-brain registrations: evaluation of three-dimensional distortions and comparison with slicewise correction. Magn. Reson. Med. 64, 1047–1056 (2010).
8. Mohammadi, S., Freund, P., Feiweier, T., Curt, A. & Weiskopf, N. The impact of post-processing on spinal cord diffusion tensor imaging. Neuroimage 70, 377–385 (2013).
9. Mohammadi, S. et al. The influence of spatial registration on detection of cerebral asymmetries using voxel-based statistics of fractional anisotropy images and TBSS. PLoS One 7, e36851 (2012). 10. De Leener, B. et al. SCT: Spinal Cord Toolbox, an open-source software for processing spinal cord MRI data. Neuroimage (2016). doi:10.1016/j.neuroimage.2016.10.009
11. Friston, K. J., Worsley, K. J., Frackowiak, R. S., Mazziotta, J. C. & Evans, a C. Assessing the significance of focal activations using their spatial extent. Hum. Brain Mapp. 1, 210–20 (1994).
Figures