0912
Regional and structural integrity of the whole cervical spinal cord using 3D-T1 MP2RAGE and multi-slice multi angle DTI and ihMT sequences at 3T: preliminary investigations on age-related changes.1Aix Marseille Univ, CNRS, CRMBM, Marseille, France, 2AP-HM, Pôle d'Imagerie Médicale, Hopital de La Timone, CEMEREM, Marseille, France, 3iLab-Spine International Associated Laboratory, Marseille, France, 4Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
In this study, we present a 3T multi-parametric MR protocol allowing structural and diffuse evaluation of the whole cervical spinal cord (SC), within a clinically acceptable scan-time. The MRI protocol includes high-resolution anatomical T2*-weighted images allowing WM/GM atrophy evaluation, a MP2RAGE sequence allowing T1-mapping, a Multi-Slice Multi-Angle (MSMA) DTI sequence allowing evaluation of tissue structural organization and, last but not least, a MSMA inhomogeneous Magnetization transfer (ihMT) sequence allowing myelin-content evaluation in whole cervical SC. This protocol was combined with a template-based automated post-processing pipeline in a preliminary study investigating age- and region-related microstructural differences in specific regions along the cervical SC.
Introduction
Quantitative MRI techniques offer tremendous perspectives for normal aging and pathological spinal cord (SC) tissue characterization [1-7]. However, limited scan time with regards to patients’ discomfort and motion artifacts usually leads to reduced spatial coverage (e.g. C2-C4), or precludes multi-parametric MRI (mp-MRI) investigations yet useful to comprehensively characterize progression and different pathophysiological patterns as encountered in amyotrophic lateral sclerosis [8, 9] or multiple sclerosis [10-12]. In this feasibility study, we present a multi-parametric MRI protocol at 3T including a prototype multi-slice-multi-angle (MSMA) Diffusion Tensor Imaging (DTI) and inhomogeneous Magnetization Transfer (ihMT) acquisitions covering C1 to C7, along with a quantitative bias-free MP2RAGE T1-mapping sequence and a high-resolution T2*-weighted imaging providing optimal GM/WM contrast for automatic segmentation purposes. As compared to promising earlier studies based on DTI and the emerging ihMT [5, 13], the total scan time (30 minutes) was greatly reduced using single-shot EPI read-outs, while offering a better spatial coverage (whole cervical cord) using MSMA, with high-spatial resolution, and an additional structural-related quantitative information (T1 relaxation time).Material and Method
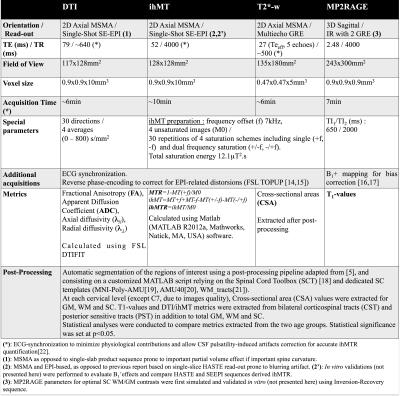
As a preliminary application of this new multi-slice mp-MRI protocol, five young (<35yo, 28.8±4.3yo, range: [22 - 34]) and five elderly (>50yo, 60.2±2.9, range: [57 - 64]) healthy subjects were scanned on a 3T system (MAGNETOM Verio; Siemens Healthcare, Erlangen, Germany) with standard coils. Main sequence parameters and post-processing are presented in Figure 1.Results and Discussion
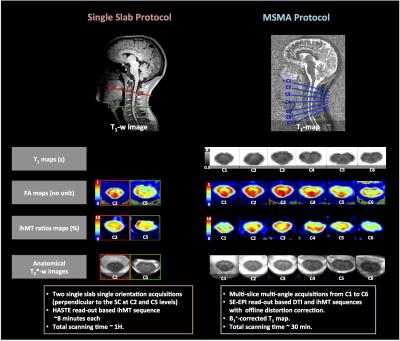
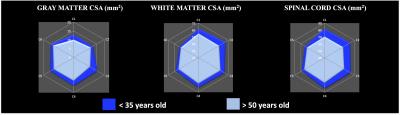
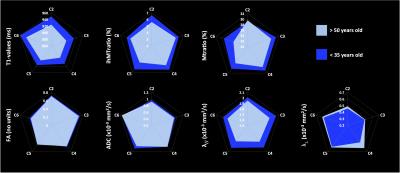
Figure 2 shows the added values of MSMA acquisitions as compared to previous single-slab single-orientation acquisitions. Figure 3 presents anatomical (GM, WM and SC CSA) metrics whereas Figure 4 summarizes mp-MRI metrics (T1, DTI and MT/ihMT) within whole WM, in the two age groups.
Interestingly, all metrics were found altered in elderly subjects. First, CSA values were significantly lower suggesting GM and WM atrophies (cf. Figure 3). Second, T1 values also exhibited a significant decrease in elderly subjects. It is worth noting that T1-values extracted from GM/WM in this study are in line with previous report investigating C3 level [23], hence adding further information on T1 distribution along the whole SC. An overall decrease tendency was observed for FA and axial diffusivity along with non-significant ADC variation. Finally, radial diffusivity was significantly increased (p<0.05), along with a significant decrease of ihMT ratios (p<0.005) and a moderate decrease of MTR, significant in lower cervical levels (p<0.005)(cf. Figure 4). All together, these variations suggest axonal loss and demyelination occurring with age consistent with previous study results [1, 4, 5].
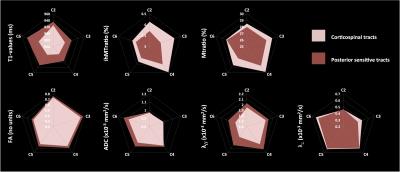
Additionally, we also found differences when comparing mp-MRI metrics within CST and PST in young subjects (cf. Figure 5): PST exhibited higher FA, ADC, axial diffusivity and T1-values and lower MT/ihMTratios and radial diffusivity as compared to CST, suggesting a higher myelination and lower axonal density of motor CST tracts as compared to posterior sensory tracts, which is consistent with previous studies [5, 24, 25].
Conclusion
In this preliminary study, we performed in vivo quantitative measurements of T1 values in the whole cervical spinal cord at 3T for the first time, compared to previous study reports on a single vertebral level [23]. We also used multi-slice multi-angle ihMT sequence for the first time, along with MSMA DTI, and were able to preliminarily characterize diffuse and regional changes of the SC microstructure related to aging or white matter pathways. Methodological improvements are under consideration in order to correct for B1 inhomogeneities and T1 relaxation effects on ihMT acquisitions. Further longitudinal follow-ups on larger cohorts are expected to confirm these preliminary but promising results. The proposed MR protocol argues for the feasibility of whole cervical SC investigation in short scan-time and its added value to future clinical investigations on degenerative SC pathologies.Acknowledgements
No acknowledgement found.References
1. Agosta, F., et al., Evidence for cervical cord tissue disorganisation with aging by diffusion tensor MRI. Neuroimage, 2007. 36(3): p. 728-35.
2. MacMillan, E.L., et al., Myelin water and T(2) relaxation measurements in the healthy cervical spinal cord at 3.0T: repeatability and changes with age. Neuroimage, 2011. 54(2): p. 1083-90.
3. Abdel-Aziz, K., et al., Age related changes in metabolite concentrations in the normal spinal cord. PLoS One, 2014. 9(10): p. e105774.
4. Wang, K., et al., Age-related changes of the diffusion tensor imaging parameters of the normal cervical spinal cord. Eur J Radiol, 2014. 83(12): p. 2196-202.
5. Taso, M., et al., Tract-specific and age-related variations of the spinal cord microstructure: a multi-parametric MRI study using diffusion tensor imaging (DTI) and inhomogeneous magnetization transfer (ihMT). NMR Biomed, 2016. 29(6): p. 817-32.
6. Hagiwara, A., et al., Utility of a Multiparametric Quantitative MRI Model That Assesses Myelin and Edema for Evaluating Plaques, Periplaque White Matter, and Normal-Appearing White Matter in Patients with Multiple Sclerosis: A Feasibility Study. AJNR Am J Neuroradiol, 2016.
7. Wheeler-Kingshott, C.A., et al., The current state-of-the-art of spinal cord imaging: applications. Neuroimage, 2014. 84: p. 1082-93.
8. Nair, G., et al., Diffusion tensor imaging reveals regional differences in the cervical spinal cord in amyotrophic lateral sclerosis. Neuroimage, 2010. 53(2): p. 576-83.
9. Cohen-Adad, J., et al., Involvement of spinal sensory pathway in ALS and specificity of cord atrophy to lower motor neuron degeneration. Amyotroph Lateral Scler Frontotemporal Degener, 2013. 14(1): p. 30-8.
10. Schlaeger, R., et al., Association Between Thoracic Spinal Cord Gray Matter Atrophy and Disability in Multiple Sclerosis. JAMA neurology, 2015. 72(8): p. 897-904.
11. Calabrese, M., et al., Exploring the origins of grey matter damage in multiple sclerosis. Nat Rev Neurosci, 2015. 16(3): p. 147-158.
12. Gilmore, C.P., et al., Spinal cord gray matter demyelination in multiple sclerosis-a novel pattern of residual plaque morphology. Brain Pathol, 2006. 16(3): p. 202-8.
13. Rasoanandrianina, H., et al. Regional and structural changes of the spinal cord tissue encountered in Amyotrophic Lateral Sclerosis (ALS): A preliminary and promising characterization using DTI and ihMT in Proceedings of the 24th annual meeting of ISMRM. 2016. Singapore, Singapore.
14. Taso, M., et al., A reliable spatially normalized template of the human spinal cord - Applications to automated white matter/gray matter segmentation and tensor-based morphometry (TBM) mapping of gray matter alterations occurring with age. Neuroimage, 2015. 117: p. 20-28.
15. Andersson, J.L., S. Skare, and J. Ashburner, How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage, 2003. 20(2): p. 870-88.
16. Smith, S.M., et al., Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage, 2004. 23 Suppl 1: p. S208-19.
17. Marques, J.P., et al., MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage, 2010. 49(2): p. 1271-81.
18. Massire, A., et al., High-resolution multi-parametric quantitative magnetic resonance imaging of the human cervical spinal cord at 7T. Neuroimage, 2016. 143: p. 58-69.
19. De Leener, B., et al., SCT: Spinal Cord Toolbox, an open-source software for processing spinal cord MRI data. Neuroimage, 2016.
20. Fonov, V.S., et al., Framework for integrated MRI average of the spinal cord white and gray matter: the MNI-Poly-AMU template. Neuroimage, 2014. 102 Pt 2: p. 817-27.
21. Levy, S., et al., White matter atlas of the human spinal cord with estimation of partial volume effect. Neuroimage, 2015. 119(0): p. 262-271.
22. Girard, O.M., et al., Magnetization transfer from inhomogeneously broadened lines (ihMT): Experimental optimization of saturation parameters for human brain imaging at 1.5 Tesla. Magn Reson Med, 2015. 73(6): p. 2111-21.
23. Smith, S.A., et al., Measurement of T1 and T2 in the cervical spinal cord at 3 tesla. Magn Reson Med, 2008. 60(1): p. 213-9.
24. Duval, T., et al., In vivo mapping of human spinal cord microstructure at 300mT/m. Neuroimage, 2015. 118: p. 494-507.
25. Duval, T., et al., g-Ratio weighted imaging of the human spinal cord in vivo. Neuroimage, 2016.
Figures