0909
Dynamic Gadoxetate Enhanced MRI measurement of segmental liver function – A novel imaging biomarker for predicting Post-Hepatectomy Liver Failure1Department of HPB and Transplantation Surgery, St James's University Hospital, Leeds, United Kingdom, 2Department of Medical Physics, St James's University Hospital, Leeds, United Kingdom, 3Department of Radiology, St James's University Hospital, Leeds, United Kingdom, 4Division of Biomedical Imaging, University of Leeds, Leeds, United Kingdom
Synopsis
Current risk assessment for post-hepatectomy liver failure (PHLF) is based on the volume of the future liver remnant (FLR). This is inaccurate when liver function is inhomogeneously distributed. We investigated whether the function of the FLR measured with DCE-MRI improves outcome predictions in 28 patients who had curative resection for colorectal liver metastases. We found the difference in preoperative estimates of FLR function and volume predicted PHLF with a sensitivity of 83% and specificity of 91%, indicating that: (1) inhomogeneous distribution of function is a major risk factor for PHLF; (2) DCE-MRI can improve patient outcome by correcting for the bias caused by volumetry.
Purpose
Major liver resection (≥3 segments) for Colorectal Liver Metastases (CRLM) carries a risk of developing Post Hepatectomy Liver Failure (PHLF). In current risk assessment CT volumetry is used to ensure the Future Liver Remnant (FLR) is adequate, but this is flawed if function is distributed inhomogeneously1. Dynamic Contrast-Enhanced MRI (DCE-MRI) with the liver-specific agent Gadoxetate is a possible solution as it can be used to measure FLR function directly2,3.
This study aimed to investigate if FLR function improves prediction of PHLF compared to FLR volume.
Methods
Data acquisition
28 patients with CRLM requiring liver resection were prospectively recruited based on their preoperative CT imaging. They underwent a preoperative staging MRI including a free-breathing DCE-MRI protocol using a 3D spoiled gradient-echo sequence (TR/TE=2.45/0.76ms, FOV=400 mm, matrix=128, FA=25°) with a 2.4s temporal resolution, 8-min acquisition, and 10ml Gadoxetate (Primovist, Bayer) delivered at 1ml/sec. Patients were followed up postoperatively using standard clinical procedures. Their biochemical and clinical progress was observed and used to determine PHLF grade according to ISGLS guidelines4.
DCE-MRI post-processing
Images were post-processed with freely available software PMI, using a dual-inlet two-compartment uptake model to interpret the temporal structure2. Arterial- and Venous Input Functions (AIF,VIF) were defined semi-automatically on a maximum signal-enhancement map, and tissue ROIs were drawn manually in each of the 8 liver segments (based on IHPBA’s definition5). The intracellular uptake rate of Gadoxetate was measured in each segment, multiplied with segmental volume, and summed over the segments in the FLR to determine FLR function, and over all segments to determine total liver function. FLR volume and total liver volume were determined from the same ROIs.
Data analysis
We test the hypothesis that subjects where the FLR volume overestimates the FLR function are more likely to develop PHLF. To quantify this, we calculate the "functional bias" defined as the difference between relative FLR function and volume:
Functional Bias = (FLR volume) / (Total liver volume) - (FLR function) / (Total liver function)
Patients were classified into two groups: “no PHLF” and “PHLF” (mild, moderate or severe) and the functional bias was compared between groups. An optimal cut-off for separation between the groups was determined with ROC analysis, and significance of differences was tested with Chi-Squared and t-test.
Results
15 patients underwent major liver resection, 8 patients underwent a more limited resection, and 6 patients were inoperable. 7 major resection patients developed mild PHLF and 4 moderate PHLF. 1 patient with a limited resection (2 segments) developed mild PHLF. 14 patients (61%) had a degree of background liver disease on their histological specimen. For 12 patients (52%) this was limited to mild steatosis; 2 patients had mild sinusoidal obstructive syndrome post-chemotherapy. Of patients who had background liver disease, 11 patients (79%) had undergone chemotherapy pre-surgery.
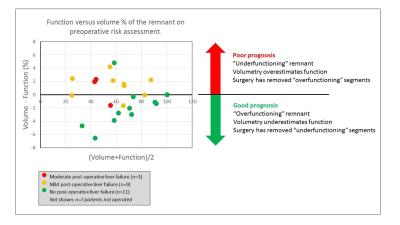
The functional bias provided a near complete separation of the groups (no PHLF/ PHLF) (Figure 1). Maximal ROC AUC of 0.85 was obtained with cut-off 0% on the functional bias, leading to a sensitivity of 83% and specificity of 91% in the prediction of PHLF. The mean functional bias was significantly different between both groups, with a lower FLR function than FLR volume for patients who developed PHLF (No PHLF: functional bias = -1.6% (1.9%), PHLF: functional bias = +1.1% (1.9%), p=0.015).
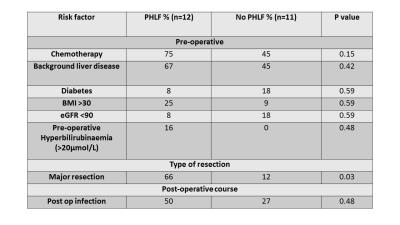
There was no statistical difference for patients who developed PHLF with pre-operative chemotherapy use or background liver disease, or other associated risk factors other than extent of resection (Figure 2).
Discussion
We found that FLR function provided a sizeable and statistically significant improvement on predicting PHLF over FLR volume alone, and that underestimated FLR function was an independent predictor of PHLF. The predictive power moreover is strong, possibly because all other known risk factors are well accounted for in the current planning process.
There was no association with PHLF and background liver disease or chemotherapy, both of which may lead to inhomogeneous liver injury distribution. There was no difference in other risk factors other than major resection, suggesting that development of PHLF could not otherwise have been anticipated.
These results suggest that DCE-MRI could be used preoperatively to improve patient selection for type of resection, and the extent of resection could be tailored more effectively to ensure adequate FLR function and improve patient outcome.
Conclusion
DCE-MRI could improve predictions of patients’ risk of PHLF by determining liver function inhomogeneity and their FLR function preoperatively. This in turn could improve the selection of patients for major resection and thereby reduce incidence of PHLF.Acknowledgements
Thanks to all the staff in the MRI department for all their help and assistance in obtaining the study DCE-MRI sequencesReferences
1. Nilsson H, Karlgren S, Blomqvist L, Jonas E. The inhomogeneous distribution of liver function: Possible impact on the prediction of post-operative remnant liver function. HPB. 2015;17(3):272–7.
2. Sourbron S, Sommer WH, Reiser MF, Zech CJ. Combined Quantification of Liver Perfusion and Function with Dynamic Gadoxetic Acid-enhanced MR Imaging. Radiology. 2012;263(3):874–83.
3. Saito K, Ledsam J, Sourbron S, Hashimoto T, Araki Y, Akata S, et al. Measuring hepatic functional reserve using low temporal resolution Gd-EOB-DTPA dynamic contrast-enhanced MRI: A preliminary study comparing galactosyl human serum albumin scintigraphy with indocyanine green retention. Eur Radiol. 2014;24(1):112–9.
4. Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: A definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149(5):713–24.
5. Strasberg SM, Phillips C. Use and dissemination of the Brisbane 2000 nomenclature of liver anatomy and resections. Ann Surg. 2013;257(3):377–82.
Figures