0905
High resolution multifrequency MR elastography of hepatic tumors for measurement of stiffness and mechanical heterogeneity1Charité - Universitätsmedizin Berlin, Berlin, Germany
Synopsis
High-resolution MRE of the liver based on multifrequency wave excitation and tomoelastography reconstruction is proposed for the mechanical characterization of hepatic tumors including stiffness and heterogeneity of the mechanical properties. We investigated 62 tumors in 43 patients and observed that high-grade tumors are stiffer than surrounding parenchyma, while low-grade hepatic adenoma had no altered stiffness. The intra-tumor heterogeneity of stiffness values was higher than in non-tumorous liver tissue. The proposed multifrequency MRE protocol could easily be integrated into the standard radiological workflow of our institution, thereby adding valuable information on tumor aggressiveness to standard clinical imaging markers.
Purpose:
Hepatic tumor detection and characterization is a major task of clinical MRI for surgery planning and therapy monitoring in hepatology. Conventional imaging biomarkers are limited in their sensitivity to microstructural properties of tumorous tissue and do not always reveal the stage and aggressiveness of the tumor. For this reason precise tumor staging still requires invasive biopsy which, however, is limited in particular for follow up investigations. In vivo MR elastography (MRE) has the potential to provide insight into the mechanical structure of hepatic tumors across many scales and may thereby offer an additional quantitative imaging marker for clinical tumor staging. However, only preliminary and partially contradicting MRE data exist on the in vivo mechanical properties of hepatic tumors [1,2]. The aim of this study was to improve the spatial resolution of stiffness maps acquired in hepatic tumors using multifrequency MRE and to measure the intra-tumor heterogeneity of the tissue’s mechanical properties.Methods:
62 tumors in 43 patients were analyzed by multifrequency MRE and tomoelastography image processing (hepatocellular carcinoma–HCC, N=15; cholangiocellular carcinoma-CCC, N=6; metastases-MET, N=35; hepatic adenoma-ADE, N=4, focal nodular hyperplasia-FNH, N=1; Hemangioma-HEM, N=1). All procedures were in accordance with the regulations of the medical ethics committee, and all patients declared their informed consent. A piezo-based actuator was used to induce time harmonic vibrations in the abdominal region at frequencies of 30, 35, 40, 45, 50, 55, and 60 Hz. MRE was performed in a 1.5-T magnet (Siemens Aera) based on a single-shot spin echo EPI sequence as detailed in [3]. 9 transversal image slices with 3x3x5 mm³ voxel size were acquired at eight time steps equally spaced over a full vibration cycle. Full wave fields were acquired at the seven vibration frequencies. Total MRE scan time was approximately 4 min under free breathing conditions. Post-processing was based on the tomoelastography pipeline which provides high resolution stiffness maps in terms of wave speed in units of m/s [4]. Statistical analysis of tumor stiffness vs. surrounding liver tissue was performed by Matlab using Wilcoxon's signed rank test for zero median.Results:
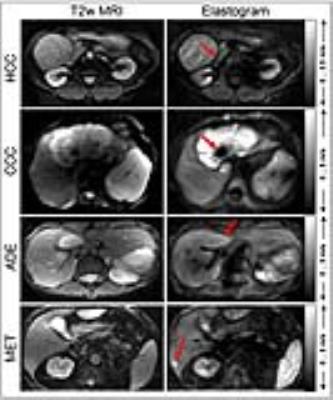
Figure 1 shows example maps of four cases in which HCC, CCC, and MET appear as stiffer masses while ADE apparently has normal properties except for slightly stiffer boundaries. The shown examples largely differ in their size and mechanical heterogeneity: while HCC has a relative homogeneous representation with slip boundaries [5], CCC shows large heterogeneity of stiffness values including necrotic areas with otherwise firm contact to surrounding tissue. Intriguingly, MET is not visible in the T2-weigthed magnitude image of the MRE scan while it appears as stiff mass in the elastogram. All entities except low-grade hepatic adenoma were significantly stiffer or showed a trend towards higher tumor stiffness as compared to surrounding liver tissue (non-tumorous tissue: 1.78±0.44 m/s vs. HCC: 2.69±0.94 m/s, P=0.083; CCC: 2.75±1.30 m/s, P=0.031; MET: 2.69±0.77 m/s, P<10-6; ADE: 1.61±0.27 m/s, P=0.625). Normalized to the surrounding liver parenchyma, the following tumor stiffness values were obtained: HCC: 1.35±0.60 m/s; CCC: 1.49±0.44 m/s; MET: 1.59±0.37 m/s; ADE: 1.09±0.21 m/s. Heterogeneity (intra-tumor standard deviation normalized by mean tumor stiffness) was significantly larger in HCC (P=0.035) and MET (P<10-5) than in non-tumorous liver tissue. Group mean values are summarized in figure 2.Discussion:
This study adds to the current understanding of in-vivo mechanical properties of hepatic tumors. High-resolution MRE allowed us to analyze local changes of stiffness inside hepatic neoplasms. Heterogeneity of stiffness seems to be a fundamental property of dysregulated tumor masses and may bear important information on the metastatic potential of tumors. As such our study could help to translate findings concerning the micromechanical properties of tumors into the macroscopic contrast of radiological images [6,7].Conclusion:
High-resolution MRE of hepatic tumors shows the high heterogeneity of the mechanical properties of liver neoplasms. Overall, high-grade tumors appear to be stiffer than surrounding parenchyma, as opposed to to low-grade hepatic adenoma which showed no significant change in stiffness. The proposed MRE protocol can easily be integrated into standard radiological workflows, thereby adding valuable information on tumor aggressiveness to standard clinical imaging markers.Acknowledgements
No acknowledgement found.References
1. Venkatesh SK, Yin M, Glockner JF, Takahashi N, Araoz PA, Talwalkar JA, Ehman RL. MR elastography of liver tumors: preliminary results. AJR Am J Roentgenol 2008;190(6):1534-1540.
2. Garteiser P, Doblas S, Daire JL, Wagner M, Leitao H, Vilgrain V, Sinkus R, Van Beers BE. MR elastography of liver tumours: value of viscoelastic properties for tumour characterisation. European radiology 2012;22(10):2169-2177.
3. Hirsch S, Guo J, Reiter R, Papazoglou S, Kroencke T, Braun J, Sack I. MR Elastography of the Liver and the Spleen Using a Piezoelectric Driver, Single-Shot Wave-Field Acquisition, and Multifrequency Dual Parameter Reconstruction. Magnetic resonance in medicine 2014;71(1):267-277.
4. Tzschatzsch H, Guo J, Dittmann F, Hirsch S, Barnhill E, Johrens K, Braun J, Sack I. Tomoelastography by multifrequency wave number recovery from time-harmonic propagating shear waves. Med Image Anal 2016;30:1-10.
5. Yin Z, Glaser KJ, Manduca A, Van Gompel JJ, Link MJ, Hughes JD, Romano A, Ehman RL, Huston J, 3rd. Slip Interface Imaging Predicts Tumor-Brain Adhesion in Vestibular Schwannomas. Radiology 2015;277(2):507-517.
6. Plodinec M, Loparic M, Monnier CA, Obermann EC, Zanetti-Dallenbach R, Oertle P, Hyotyla JT, Aebi U, Bentires-Alj M, Lim RY, Schoenenberger CA. The nanomechanical signature of breast cancer. Nat Nanotechnol 2012;7(11):757-765.
7. Leal-Egana A, Fritsch A, Heidebrecht F, Diaz-Cuenca A, Nowicki M, Bader A, Kas J. Tuning liver stiffness against tumours: An in vitro study using entrapped cells in tumour-like microcapsules. Journal of the mechanical behavior of biomedical materials 2012;9:113-121.
Figures