0904
Respiratory resolved, self-gated, 4-dimensional MRI using Rotating Cartesian K-Space (ROCK): technical validation and initial clinical experience on an MRI-guided radiation therapy system1Radiology, University of California, Los Angeles, CA, United States, 2Radiation Oncology, University of California, Los Angeles, CA, United States
Synopsis
Respiratory revolved 4D-MRI is used to quantify patient-specific respiratory motion to ensure optimal dose delivery in the radiation therapy of abdominal tumors. In this work, we developed a 4D-MRI technique using 3D k-space encoding, respiratory motion
Introduction
In radiation therapy of abdominal tumors, respiratory revolved 4D-MRI is used to quantify patient-specific respiratory motion for optimal radiation dose delivery1. Most existing 4D-MRI methods are based on retrospective sorting of 2D real-time images and therefore suffer from limited spatial resolution and stitching artifacts which potentially undermine tumor delineation and motion quantification2,3. To address these issues, we developed and evaluated a self-gated 4D-MRI technique based on 3D k-space binning.
Method
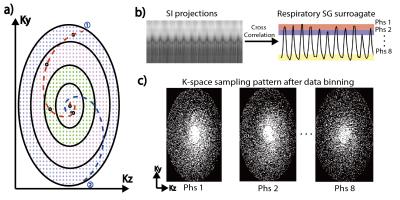
A 3D balanced SSFP sequence was modified with Rotating Cartesian K-space (ROCK) sampling pattern4. As shown in Fig.1a, the Cartesian grid was reordered in a quasi-spiral fashion with successive spiral arms rotated using golden angle to ensure uniform k-space sampling after retrospective data binning. Each quasi-spiral arm started with the k-space center-line, which were used for motion self-gating. The k-space center-lines were Fourier transformed into projections. Subsequently, the respiratory self-gating signal was calculated by maximizing the cross-correlation coefficient of each projection with the reference (i.e. first projection). The derived self-gating signal was used to bin the k-space data into 8 respiratory phases (Fig.1b). Finally, dynamic 3D images were reconstructed using:
$$f(x)=argmin_x\sum_i\left\{||D_iFSx_i-y_i||_2^2+\lambda\Psi(x_i)\right\}+\lambda_m\mathcal{R}(x)$$
where $$$x_i$$$ is the reconstructed images; $$$D$$$ and $$$F$$$ represent under-sampling and Fourier operations; $$$S$$$ is the coil sensitivity map estimated by ESPIRiT5; $$$y_i$$$ is the k-space measurements; $$$\Psi(x_i)$$$ and $$$\mathcal{R}(x)$$$ are regularization terms in the spatial and respiratory dimensions.
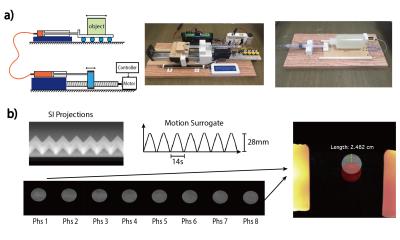
Phantom Study: The proposed 4D-MRI was validated in a 1.5T scanner using a custom-made MRI-compatible motion phantom (Fig.2a). The triangular motion waveform was programmed with different magnitudes (28, 14mm) and periods (8s, 12s, 16s, 20s). The 4D-MRI scan was performed for each motion configurations and the locations of the object in each respiratory position were measured and compared with the ground truth. Important sequence parameters: FOV=500x300x200mm; resolution=1.2x1.2x1.6mm; TE/TR=2.0/4.0ms; flip angle=65; scan time=5min; fat saturation every 200ms.
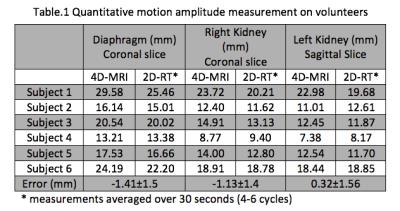
Volunteer Study: The proposed 4D-MRI was also tested in 6 healthy volunteers. No specific breathing instructions were given. 4D-MRI sequence parameters were identical with those used in phantom experiment. Additional 2D real-time (2D-RT) scans were repeated in different orientations (bSSFP, resolution=1.8x1.8x6mm; 5fps; scan time=30s). The reconstructed 4D images were reformatted to match the three orthogonal planes of 2D-RT and in-plane motion amplitudes of three ROI (diaphragm, left and right kidney) were measured and compared. The motion amplitudes of multiple cycles of the 2D-RT images were averaged as the final measurements.
Patient Study: The proposed 4D-MRI was also implemented on a 0.35T MRI-guided radiation therapy (MGRT) system (MRIdian, ViewRay, Cleveland, OH). The following parameters were optimized to compensate the SNR penalty of the low field strength: flip angle=120; TE/TR=2.8/5.6; scan time=7min. The study included 4 patients with liver cancer and 4D-MRI was performed right after their treatment in the MGRT system, with no specific breathing instructions. The reconstructed images were evaluated against the 4D-CT.
Result
Fig.2 shows the result of phantom experiment, where motion surrogate derived from k-space centerlines was in accordance with the ground truth (amplitude=28mm, period=14s). The images of the first and last respiratory positions are color-overlaid for motion amplitude measurement. Motion amplitude measurements under different configurations were 11.89% smaller than the ground truth, whereas a difference of 12.5% was expected due to the binning effect.
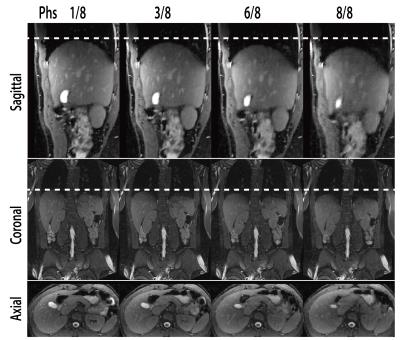
Fig.3 shows the reconstructed 4D-MRI images on a volunteer. Major abdominal organs, including the liver, kidneys, and pancreas are clearly visualized in 3D and in different respiratory positions. Quantitative in vivo motion amplitude measurements over three ROIs are listed in Table 1. The measurements based on 4D-MRI were within 1.5mm of those based on 2D-RT reference standard, which could be due to the intra-scan and inter-scan variation of the breathing pattern and the relatively low spatial resolution of 2D-RT images.
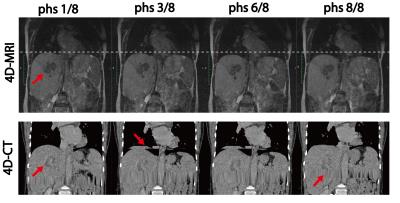
Fig.4 shows the 4D-MRI images of a patient with liver tumor, acquired in the 0.35T MGRT. Comparing with the 4D-CT images of the same patient, the 4D-MRI images have much better soft tissue contrast for tumor visualization and are free of the stitching artifacts seen in 4D-CT images.
Conclusion
The proposed 4D-MRI technique could provide high resolution, high quality, respiratory motion resolved 4D images with good soft-tissue contrast and are free of the “stitching” artifacts usually seen on 4D-CT and most existing 4D-MRI based on 2D image sorting. Results from motion phantom, volunteer and patient studies in two different field strengths validated the feasibility of using the proposed 4D-MRI technique to quantify abdominal organ motion in MRI-based radiation treatment planning.Acknowledgements
The authors acknowledge funding support from the National Institutes of Health under the award numbers R01HL127153 and R01CA188300. The authors would also like to thank Yuanfei Jiang, for her help in the design and implementation of the MRI-compatible motion phantom.References
1. Cai J, Chang Z, Wang Z, Paul Segars W, Yin F-F. Four-dimensional magnetic resonance imaging (4D-MRI) using image-based respiratory surrogate: A feasibility study. Medical Physics. 2011;38(12):6384.
2. Du D, Caruthers SD, Glide-Hurst C, Low DA, Li HH, Mutic S, Hu Y. High quality t2-weighted 4D magnetic resonance imaging for radiation therapy applications. International journal of radiation oncology, biology, physics. 2015;92(2):430–437.
3. Deng Z, Pang J, Yang W, Yue Y, Sharif B, Tuli R, Li D, Fraass B, Fan Z. Four-dimensional MRI using three-dimensional radial sampling with respiratory self-gating to characterize temporal phase-resolved respiratory motion in the abdomen. Magnetic Resonance in Medicine. 2016;75(4):1574–85.
4. Han F, Zhou Z, Han E, Gao Y, Nguyen K-L, Finn JP, Hu P. Self-gated 4D multiphase, steady-state imaging with contrast enhancement (MUSIC) using rotating cartesian K-space (ROCK): Validation in children with congenital heart disease. Magnetic resonance in medicine. 2016 Aug 16.
5. Uecker M, Lai P, Murphy MJ, Virtue P, Elad M, Pauly JM, Vasanawala SS, Lustig M. ESPIRiT-an eigenvalue approach to autocalibrating parallel MRI: Where SENSE meets GRAPPA. Magnetic Resonance in Medicine. 2013;1001:990–1001.
Figures