0902
Comprehensive T1-weighted dynamic liver MRI during free-breathing using fat/water separation, radial sampling, compressed sensing, parallel imaging, and motion-weighted reconstruction1Center for Advanced Imaging Innovation and Research (CAI2R), Department of Radiology, New York University School of Medicine, New York, NY, United States, 2Bernard and Irene Schwartz Center for Biomedical Imaging, Department of Radiology, New York University School of Medicine, New York, NY, United States
Synopsis
Conventional clinical liver MRI consists of several exams, including pre-contrast in-phase, opposed-phase, and fat-saturated scans as well as multiple scans with contrast-enhancement. For each of these acquisitions, accurate breath-holding is required to ensure diagnostic image quality.
Here, we demonstrate how this entire protocol can be replaced by using a single comprehensive exam, where only one dataset has to be acquired during free-breathing. All relevant images can be retrospectively generated with model-based fat/water separation, which incorporates compressed sensing and parallel imaging. This approach has the potential to improve clinical workflow and eliminate the risk for failed exams caused by imperfect breath-holding.
Purpose
Conventional T1-weighted clinical liver MRI consists of several separate exams, which all rely on accurate breath-holding (BH). Especially for sick, elderly, or pediatric patients, however, breath-holding can fail and, therefore, image quality can be severely reduced.
To overcome this problem, techniques for free-breathing DCE-MRI have been developed1,2, where all data are acquired continuously and a dynamic image series is reconstructed retrospectively. Most of these approaches rely on spectral fat saturation, which has two limitations: 1) Fat signal can remain visible in regions with B0 field inhomogeneities, which reduces the overall image quality. 2) Conventional non-fat-suppressed scans have to be acquired separately to assess fat content, which again requires breath-holding and increases the exam time.
Here, we demonstrate how both problems can be resolved simultaneously by using a single continuous free-breathing radial acquisition. A model-based fat/water separation technique is employed to extract dynamic fat and water series. Subsequently, all T1-weighted contrasts required for a clinical liver exam can be generated with this one-stop-shop approach.
Methods
Data acquisition
In this IRB-approved prospective study, five healthy subjects (age 32.0±9.5 years), underwent two separate liver examinations using a 3T scanner (Magnetom Prisma, Siemens Healthineers). Each session included the injection of a half dose of contrast agent (Magnevist, Bayer Healthcare, Germany). In one session, a slightly modified version of the conventional clinical abdominal protocol was performed, including a pre-contrast BH-Dixon scan and contrast-enhanced BH-VIBE acquisitions to capture the arterial, venous, and equilibrium enhancement phases. In the other session, a single comprehensive free-breathing acquisition using the RAVE (RAdial Volumetric Encoding) sequence3 was performed for 4:52min. Here, contrast agent was injected 30s after the start of the sequence. Data were acquired continuously with a 3D stack-of-stars trajectory and bipolar multi-echo readout. To improve temporal incoherence, blip gradients were inserted between the readout events, which rotate subsequent projections by 2 degrees. Imaging parameters are shown in Figure 1.
Image reconstruction
Both conventional BH-Dixon and BH-VIBE datasets were reconstructed with the vendor-provided techniques. For RAVE datasets, 34 consecutive spokes were grouped into a single temporal frame, yielding 29 frames with a temporal resolution of 9.9 s each. To obtain the dynamic water and fat series, a modified version of the Dixon-RAVE optimization problem3 was solved:
$$\text{argmin}\sum_{c,t}\|\Lambda\left(E(W,F)_{c,t}-y_{c,t}\right)\|_2^2+\lambda_W\|S(W)\|_1+\lambda_F\|S(F)\|_1$$
$$$y$$$ are 4D k-space data (three spatial dimensions and one temporal dimension), $$$W$$$ and $$$F$$$ are 4D time-resolved water and fat maps. Compressed sensing is incorporated by using temporal finite differences as sparsifying transform $$$S$$$. The forward operator $$$E$$$ transforms the to-be-estimated parameters water and fat to k-space data.
In contrast to previous work3, the precomputed field map4 was assumed to be constant for all dynamic frames. For most cases, this is justified because of its spatial smoothness. With this simplification, the signal model becomes a linear instead of a non-linear function, which allows using a faster optimization algorithm (e.g., lBFGS).
A respiratory curve was extracted from the k-space data and used to further improve the motion robustness. Here, motion-weighting of the data-fidelity term was performed and implemented as weighting matrix $$$\Lambda$$$ with weights following an exponential decay5.
From the reconstructed water and fat image series, in-phase and opposed-phase images can be synthetically generated. Dixon-RAVE images were compared to corresponding conventional images, as shown in Figure 1.
Reader evaluation
Results were scored by two blinded experienced radiologists on a 5-point scale using the following categories: Overall image quality, liver edge sharpness and hepatic vessel clarity, streak artifact, and degree of fat suppression. Scores from both readers and the different extracted contrasts were averaged. Wilcoxon signed rank tests were used for statistical evaluation.
Results
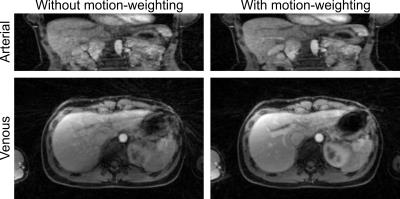
Figure 2 shows the effect of including motion-weighting in the reconstruction. Compared to the non-motion-weighted reconstruction, residual blurring is reduced and vessel sharpness is increased.
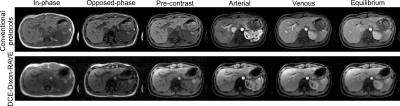
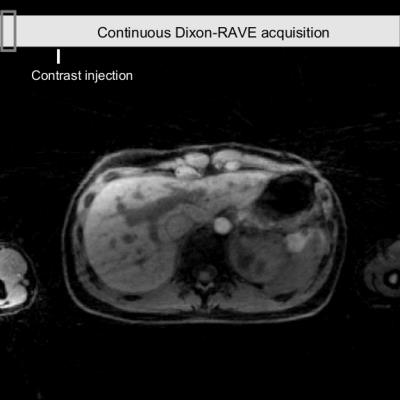
Figure 3 shows the comparison between conventional breath-held imaging protocols (first row) and the proposed comprehensive free-breathing exam. Figure 4 shows the entire dynamic image series, which is acquired and reconstructed with the proposed approach.
Scores from the reader study are shown in Figure 5.
Discussion and Conclusion
This work demonstrates how a single continuous free-breathing radial acquisition in combination with a model-based reconstruction can be utilized to generate all clinically needed T1-weighted images for liver MR examination. Diagnostic image quality was achieved with the comprehensive Dixon-RAVE approach, although results with the conventional protocols were better. This finding, however, is expected to invert when patients with poor breath-holding capability are scanned instead of healthy volunteers.
In summary, Dixon-RAVE has the potential to increase patient comfort, improve clinical workflow, and eliminate the risk for failed exams caused by imperfect breath-holding.
Acknowledgements
NIH P41EB017183, 5R01EB018308
References
1. Feng L et al., Golden-angle radial sparse parallel MRI: Combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI, Magn Reson Med, 72:707-717 (2014)
2. Zhang T et al., Fast pediatric 3D free-breathing abdominal dynamic contrast enhanced MRI with high spatiotemporal resolution, J Magn Reson Imag, 41:460-473 (2015)
3. Benkert T et al., Free-breathing volumetric fat/water separation by combining radial sampling, compressed sensing, and parallel imaging, Magn Reson Med, 10.1002/mrm.26392 (2016)
4. Liu J et al., Method for B0 off-resonance mapping by non-iterative correction of phase-errors (B0-NICE), Magn Reson Med, 74:1177-1188 (2015)
5. Cheng JY et al., Free-breathing pediatric MRI with nonrigid motion correction and acceleration, J Magn Reson Imag, 42:407-420 (2015)
Figures