0901
Altered Functional Network Topology in Children with Complex Congenital Heart Disease is Associated with Reduced Regional Cerebral Perfusion and Cognitive Outcomes1Radiology, Children's Hospital of Pittsburgh of UPMC, Pittsburgh, PA, United States, 2University of Pittsburgh
Synopsis
Pseudo-continuous ASL (pCASL) and resting-state BOLD data was obtained from a cohort of pre-adolescent patients with complex congenital heart disease (CHD) and healthy controls. CHD patients displayed altered functional network topology (network segregation) in posterior default mode, subcortical, and prefrontal regions, as well as decreased regional CBF in subcortical and anterior default mode regions. These alterations in functional topology and regional CBF were also associated with deficits in cognitive performance as measured by the NIH Toolbox (crystallized cognition). These results suggest regional alterations in neuronal-vascular coupling may underlie neurocognitive deficits in pre-adolescent CHD patients.
Purpose
Complex congenital heart disease (CHD) is associated with altered functional network topology1 and altered cerebral metabolism2-4 in the fetal and neonatal periods, postulated to be the cause of long term neurocognitive deficits5,6. We tested the hypothesis that older children with CHD would demonstrate altered functional connectivity associated with hypometabolism and that these relationships would be predictive of cognitive function.Materials and Methods
A total of 84 pediatric/adolescent subjects (44 complex CHD and 40 controls) were prospectively recruited with a total of 74 completed MRI scans and NIH Tool Box neurocognitive assessment. Participants were scanned on a Siemens 3T Skyra system using a 32 channel head coil. For ASL, pseudo-continuous ASL (pCASL) imaging was used with labeling duration = 1500 ms, PID = 1200 ms, EPI matrix = 64 X 64, in-plane resolution = 4 X 4 mm, slice thickness = 4 mm, total acquisition time = 6 min. For BOLD, standard EPI was used with TR = 2 s, TE = 33 ms, in-plane resolution = 4 X 4 mm, slice thickness = 4 mm, two runs acquired of 5 min. each.
BOLD Analysis: Data from 18 complex CHD patients (age = 14.36 ± 2.6 years) and 29 healthy controls (13.4 ± 4.2 years) were included in the analysis after motion correction. Previously validated methods7,8 were used to ensure robustness to motion artifacts, including slice timing correction, motion correction, and normalization to the AAL template9. Time courses were extracted from the 90 cortical regions in the AAL parcellation atlas and low-pass filtered (0.008 Hz < f < 0.08 Hz). Volumes were censored for framewise displacement > 0.2 mm or DVARS > 25. Nuisance regressors, including motion parameters, global signal, and drift correction, were regressed out. Correlation matrices were computed and thresholded at various values of cost ranging from 0.05 to 0.4. The Brain Connectivity Toolbox (BCT; Indiana University, Bloomington, IN) toolbox was used for computation of global and nodal graph metrics at each value of cost. Metrics were compared using a mixed-effects GLM with sex and age as covariates of no interest, to properly account for between- and within-participant variability.
ASL Analysis: Data from 23 complex CHD patients (age = 13.4 ± 3.8 years) and 33 healthy controls (age = 14.8 ± 4.1 years) were included in the analysis. Data was spatially coregistered to MNI space using routines in SPM8. Absolute CBF was estimated using the two-compartment model10 with literature parameter values11 and compared on a voxelwise basis using a GLM with sex and age as covariates of no interest. A Monte Carlo simulation was used to determine regions with altered CBF with FWE-corrected p < 0.05.
Results
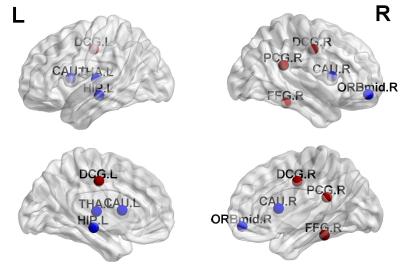
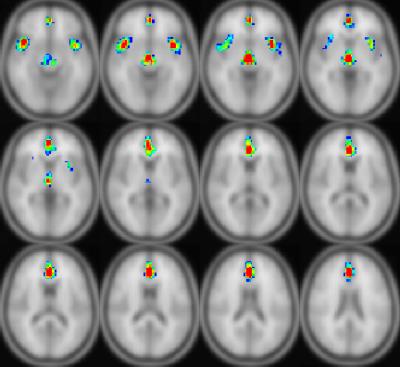
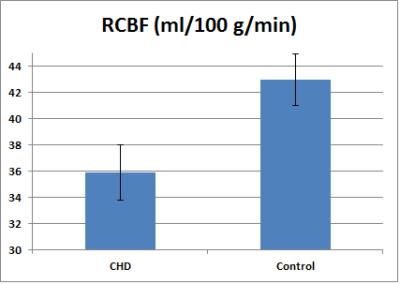
Older children with complex CHD show regional differences in functional network topology compared to the healthy control group, with increased local efficiency (segregation) in posterior default mode regions (DMN) but decreased local efficiency (segregation) in subcortical and prefrontal regions (Figure 1). Decreased regional CBF (ASL imaging) was noted in the complex CHD group compared to the healthy control group in the insula, thalamus, and anterior cingulate (anterior DMN) bilaterally (Figure 2). The reduction is substantial, almost 20% in the right insula (peri-opercular) (Figure 3).
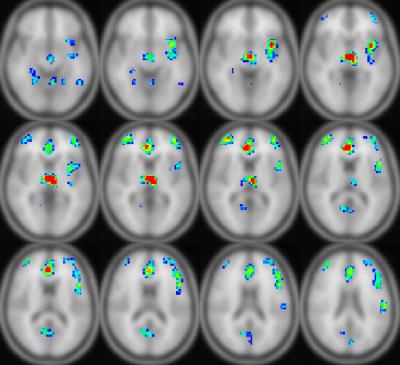
Correlations with cognitive measures were also found. Local efficiency in the posterior cingulate positively correlates with the crystallized composite cognition measure from the NIH Toolbox (CCC); additionally, eigenvector centrality negatively correlates in subcortical regions but positively in the posterior cingulate (Figure 4). Regional CBF in these regions, in addition to prefrontal regions and the posterior cingulate, positively correlates with the CCC (Figure 5).
Discussion
Alterations in structural network topology in older children with CHD have been previously seen12, which were shown to mediate poorer neurocognitive performance. Here, we show that CHD is also associated with altered functional network topology with abnormal segregation of anterior cingulate, pre-cuneus/posterior cingulate, subcortical, and prefrontal networks. There is overlap of these findings with hypometabolism as represented by reduced regional CBF in subcortical and anterior default mode regions. These regional vulnerabilities overlap with the salience/cingular-opercular and default-mode resting state networks that undergo critical maturation during the pre-adolescent period and support the development of executive control during the transition to adulthood13. These findings suggest that critical regional maturational deficits in neuronal vascular coupling are present in CHD patients and may underlie neurocognitive deficits.Conclusion
Older children with complex CHD demonstrate functional connectivity alterations that overlap with hypometabolism as delineated by reduced regional CBF. These findings suggest that regional maturational deficits in neuronal-vascular coupling may underlie neurocognitive deficits in pre-adolescent CHD patients.Acknowledgements
No acknowledgement found.References
1. Schmithorst VJ, Lee V, Votava-Smith J, Sulaiman S, Ceschin R, Paquette L, et al. Complex Congenital Heart Defects in Infants Produce Lasting Decreases in Functional Network Segregation. ISMRM 24th Annual Meeting, Singapore. 2016.
2. Sun L, Macgowan CK, Sled JG, Yoo SJ, Manlhiot C, Porayette P, et al. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation. 2015;131(15):1313-23.
3. Nagaraj UD, Evangelou IE, Donofrio MT, Vezina LG, McCarter R, du Plessis AJ, et al. Impaired Global and Regional Cerebral Perfusion in Newborns with Complex Congenital Heart Disease. The Journal of pediatrics. 2015;167(5):1018-24.
4. Jain V, Buckley EM, Licht DJ, Lynch JM, Schwab PJ, Naim MY, et al. Cerebral oxygen metabolism in neonates with congenital heart disease quantified by MRI and optics. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2014;34(3):380-8.
5. Cheng HH, Wypij D, Laussen PC, Bellinger DC, Stopp CD, Soul JS, et al. Cerebral blood flow velocity and neurodevelopmental outcome in infants undergoing surgery for congenital heart disease. The Annals of thoracic surgery. 2014;98(1):125-32.
6. Hoffman GM, Brosig CL, Mussatto KA, Tweddell JS, Ghanayem NS. Perioperative cerebral oxygen saturation in neonates with hypoplastic left heart syndrome and childhood neurodevelopmental outcome. The Journal of thoracic and cardiovascular surgery. 2013;146(5):1153-64.
7. Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage. 2014;84:320-41.
8. Power JD, Schlaggar BL, Petersen SE. Recent progress and outstanding issues in motion correction in resting state fMRI. NeuroImage. 2015;105:536-51.
9. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15(1):273-89.
10. Alsop DC, Detre JA. Reduced transit-time sensitivity in noninvasive magnetic resonance imaging of human cerebral blood flow. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1996;16(6):1236-49.
11. Schmithorst VJ, Hernandez-Garcia L, Vannest J, Rajagopal A, Lee G, Holland SK. Optimized simultaneous ASL and BOLD functional imaging of the whole brain. Journal of magnetic resonance imaging : JMRI. 2014;39(5):1104-17.
12. Panigrahy A, Schmithorst VJ, Wisnowski JL, Watson CG, Bellinger DC, Newburger JW, et al. Relationship of white matter network topology and cognitive outcome in adolescents with d-transposition of the great arteries. NeuroImage Clinical. 2015;7:438-48.
13. Marek S, Hwang K, Foran W, Hallquist MN, Luna B. The Contribution of Network Organization and Integration to the Development of Cognitive Control. PLoS biology. 2015;13(12):e1002328.
Figures