0899
Brain metabolic rate, but not perfusion or brain volume, predicts clinical scores in newborns at risk for brain injury1Radiology, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2Radiology, Shengjing hospital of China Medical University, Shenyang, People's Republic of China, 3Imaging Systems Clinical Science, Philips Healthcare, Beijing, People's Republic of China
Synopsis
Hypoxic brain injury due to perinatal oxygen deprivation is one of the leading reasons of neonatal death and long-term disabilities. In this study, we evaluated the predictive values of cerebral blood flow (CBF), oxygen extraction fraction (OEF), cerebral oxygen metabolism (CMRO2), and brain volume as biomarkers in the assessment of hypoxic brain injuries in neonatal patients. Our results showed that among these biomarkers, only CMRO2 was significantly associated with Apgar score, which is the standard clinical score indicating the risks of prenatal and perinatal brain injuries. CBF and brain volume increase with age, but have no relationship with Apgar score.
Purpose
Hypoxic brain injury due to perinatal oxygen deprivation is one of the leading reasons of neonatal death and long-term disabilities (1). Functional biomarkers such as brain metabolic rate are expected to provide earlier and more sensitive predictions of clinical outcomes, compared to conventional structural imaging. However, due to the lack of techniques, little is known about these biomarkers in neonatal hypoxic injury. In this work, we applied a recently developed non-invasive MRI techniques in neonates with hypoxic injuries to measure their cerebral blood flow (CBF), oxygen extraction fraction (OEF) and cerebral oxygen metabolism (CMRO2), and compared them with clinical assessment in neonatal function(Apgar score). We aimed to examine the predictive values of these biomarkers in evaluating hypoxic brain injuries in neonatal patients.Methods
Subjects: We studied 53 consecutive neonates who had MRI due to clinical indication of hypoxic brain injury (38 males, age at birth 34.2±3.7 gestational weeks, age at scan 36.4±2.9 gestational weeks). Apgar scores, which is the standard assessment of the clinical status of newborns immediately after delivery, were collected. MRI data were collected on a 3T Philips system. All data were used upon institutional ethics committee approval. Clinical diagnosis was based on the standard T1 and T2 images following standard practice.
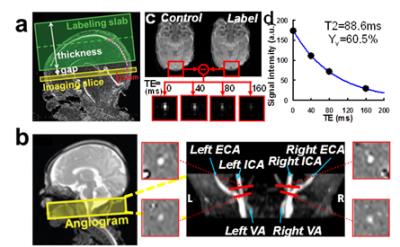
OEF was measured using a T2-relaxation-Under-Spin-Tagging(TRUST) sequence which was recently adapted to neonates (2). The TRUST MRI measured global venous oxygenation (Yv) non-invasively at superior sagittal sinus (Figure 1a). OEF was then calculated by OEF=(Ya-Yv)/Ya, where arterial oxygenation (Ya) was measured by pulse oximetry.
CBF was measured by phase-contrast MRI that was optimized previously (3)(Figure 1b). Whole-brain CBF was quantified (in ml/100g/min) by normalizing the total blood flux that enters the brain to brain volume acquired from anatomic T2 images.
CMRO2 was then calculated (in umol/100g/min) using the Fick principle, i.e., CMRO2=CBFx(Ya-Yv)xCa, where Ca is the amount of oxygen that a unit volume of blood can carry (8.97μmol O2/100ml blood).
Statistical analysis: Regression analysis was used to evaluate the relationship between the MRI measures (OEF, CBF, CMRO2 and brain volume) and clinical assessment (1-min Apgar and 5-min Apgar), with age and gender as covariates. We considered both the age at scan, and the age at birth with time after birth. Multiple comparison-corrected p<0.05 is considered significant.
Results
In the 53 neonates scanned, 22 had normal anatomic appearance. 17 had punctate while matter lesion (PWML), 4 had primary subarachnoid hemorrhage, and 10 had neonatal encephalopathy. 1-min and 5-min Apgar scores were 7.8±2.4 and 8.7±2.1, respectively. The Apgar scores showed no correlation with age at birth and gender. OEF, CBF, CMRO2 and brain volume of the group were 29.2±10.0%, 16.3±9.3ml/100g/min, 34.0±14.7μmol/100g/min, and 310.1±63.8mm3, respectively. These values are within the range from previous literature using other imaging methods (4-6).
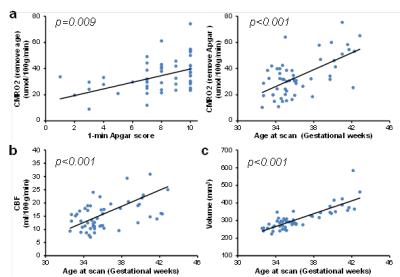
Using age at scan, gender and Apgar score (1-min or 5-min) as independent variables, regression analysis showed that CMRO2 showed significant positive correlations with age (p<0.001) and Apgar scores (p=0.009 for 1-min Apgar, p=0.027 for 5-min Apgar)(Figure. 2a). Both CBF and brain volume showed a significant positive correlation with age (Figure 2b and c, p<0.001 for both) but not with the Apgar scores(p>0.32). The age-dependence of CMRO2, CBF and brain volume is also in agreement with literature (2,5,6). OEF showed no dependence on age and the Apgar scores. Moreover, none of these parameters showed a significant gender-dependence. These relationships remained significant when we used age at birth and time after birth to replace age at scan in the regression analysis.
Discussion
A significant finding of this study is the association between CMRO2 and Apgar score. Since low Apgar scores correlate with prenatal and perinatal adversities, this association suggested that CMRO2 may be a sensitive biomarker in detecting hypoxic brain injuries in neonates. We further conducted an exploratory analysis in a sub-group of 22 neonates whose anatomic images did not show any abnormalities. CMRO2 also showed a trend of significant correlation with 1-min Apgar score (p=0.07), despite the narrow range of Apgar score (from 7 to 10). On the other hand, it has been reported that low Apgar score confers increased risks of neonatal and infant death and with long-term neurological disability (e.g., cerebral palsy, epilepsy and cognitive impairment)(7). Therefore, it is plausible that CMRO2 may also be associated with long-term clinical outcomes of these neonates. Future studies will be performed to evaluate this hypothesis.Conclusion
We measured cerebral hemodynamics and metabolism non-invasively using MRI in neonates with hypoxic injury. Our results suggested that CMRO2 is a more sensitive marker in assessing the extent of brain injury, compared to cerebral perfusion and brain volume.Acknowledgements
No acknowledgement found.References
1. Lawn JE, Cousens S, Zupan J, Lancet Neonatal Survival Steering T. 4 million neonatal deaths: when? Where? Why? Lancet 2005; 365(9462): 891-900.
2. Liu P, Huang H, Rollins N, Chalak LF, Jeon T, Halovanic C, Lu H. Quantitative assessment of global cerebral metabolic rate of oxygen (CMRO2) in neonates using MRI. NMR Biomed 2014; 27(3): 332-40.
3. Liu P, Qi Y, Lin Z, Zhao X, Guo Q, Wang X, Lu H. Toward routine assessment of cerebral blood flow in neonates and infants: a phase-contrast MRI study. In: Proceedings of ISMRM 24th Annual Meeting. Singapore, 2016. p 559.
4. Jain V, Buckley EM, Licht DJ, Lynch JM, Schwab PJ, Naim MY, Lavin NA, Nicolson SC et al. Cerebral oxygen metabolism in neonates with congenital heart disease quantified by MRI and optics. J Cereb Blood Flow Metab 2014; 34(3): 380-8.
5. De Vis JB, Petersen ET, Alderliesten T, Groenendaal F, de Vries LS, van Bel F, Benders MJ, Hendrikse J. Non-invasive MRI measurements of venous oxygenation, oxygen extraction fraction and oxygen consumption in neonates. Neuroimage 2014; 95: 185-92.
6. Huppi PS, Warfield S, Kikinis R, Barnes PD, Zientara GP, Jolesz FA, Tsuji MK, Volpe JJ. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann Neurol 1998; 43(2): 224-35.
7. Ehrenstein V. Association of Apgar scores with death and neurologic disability. Clin Epidemiol 2009; 1: 45-53.
Figures