0898
DTI reveals crucial white matter lesions in bilateral spastic cerebral palsy in infants with non-cystic periventricular leukomalaciaHaoxiang Jiang1,2,3, Xianjun Li1,2, Chao Jin1, Miaomiao Wang1, Congcong Liu1, Kevin C. Chan4, and Jian Yang1,2
1Department of Diagnostic Radiology, The First Affiliated Hospital, Xi’an Jiaotong University, Xi’an, People's Republic of China, 2Department of Biomedical Engineering, School of Life Science and Technology, Xi’an Jiaotong University, Xi’an, People's Republic of China, 3Department of Diagnostic Radiology, Xi’an Children Hospital, Xi’an, People's Republic of China, 4Departments of Ophthalmology and Bioengineering, University of Pittsburgh, PA, United States
Synopsis
Predicting cerebral palsy (CP) in infants with periventricular leukomalacia (PVL) is important for early treatment and rehabilitation. This study aimed to determine the crucial WM lesions in spastic CP (SCP) of non-cystic PVL infants using diffusion tensor imaging (DTI). Our results suggest that there was deceased FA in the corticospinal tract (CST) of bilateral SCP infants, but not in PVL infants without CP. Meanwhile, the posterior thalamic radiation, and the splenium of corpus callosum were damaged both in SCP and No-CP PVL infants. Therefore, Lower FA in the CST maybe a prerequisite and biomarker for identifying and predicting the outcome of SCP in infants with non-cystic PVL.
Introduction
Periventricular leukomalacia (PVL) is a major form of white matter (WM) injury due to hypoxic-ischemic encephalopathy, etc1.There are two types of PVL (cystic and non-cystic). Compared with cystic PVL, a low morbidity of CP was found in non-cystic PVL2,3 Up to now, the accurate prediction of CP outcome is still a big challenges in non-cystic PVL infants. However, early CP diagnosis is important for early treatment strategies. Therefore, this study aims to determine the crucial WM lesions in spastic cerebral palsy (SCP) in non-cystic PVL infants using diffusion tensor imaging (DTI).Methods
15 PVL infants with SCP, 11 non-spastic PVL infants, and 17 normal control infants were retrospectively enrolled. The children with SCP were diagnosed by two pediatric neurologists. MR examinations were performed between the ages of 6–18 months using a 3T scanner (GE, Signa HDxt) with an 8-channel head coil. The protocols sequence included 3D-MPRAGE T1WI(TR/TE, 10ms/4.6ms; matrix, 256×256; section-thickness, 1mm; FOV, 240mm), FSE-T2WI (TR/TE, 6500ms/124ms; matrix, 256×256; section-thickness, 4mm; FOV, 180mm) and DTI(35 directions; b-value, 1000 s/mm2; TR/TE, 5500ms/95ms; section-thickness,4mm; FOV, 180mm, matrix, 256×256). DTI data was processed with the aid of the FMRIB software library (FSL, www.fmrib.ox.au.uk/fsl). TBSS was used to align FA images of all subjects to the target image. The mean FA image and its skeleton were created. Each subject’s aligned FA images were projected onto the mean FA skeleton (threshold=0.2). Voxel-wise, cross-subject statistics was performed to assess differences in FA between bilateral SCP, No-CP, and control groups. Additionally, automated fiber-tract quantification (AFQ) software tools were used to identify WM tracts in each participant's brain (https://github.com/jyeatman/AFQ). The FA values along the core fiber which are plotted for 100 equidistant locations between two defining ROIs were compared between the groups. All statistical analysis were performed by using SPSS 17.0 and p<0.05 was considered as statistically significant difference.Results
Group comparison of FA values between bilateral SCP, No-CP, and control groups were shown by TBSS maps(Fig.1). Compared with the control group, there was a significant decrease in FA in widespread areas of white matter in the PVL infants with SCP, while the FA decrease was mainly in the posterior white matter of the non-spastic infants (P<0.05). Compared with the non-spastic PVL infants, the SCP infants showed significantly lower FA values in regional WM, including bilateral cerebral peduncle, posterior limb of the internal capsule, corona radiata, external capsule, genu and splenium of corpus callosum (P<0.05).The AFQ results showed a decrease in FA in the bilateral corticospinal tract (CST) only in infants with SCP. The other tract profiles (posterior thalamic radiation, genu, and the splenium of corpus callosum) showed decreased FA values in both the bilateral SCP and No-CP groups (P<0.05) (Fig.2).Discussion
This study found that PVL infants with bilateral SCP showed significantly lower FA values in regional WM, e.g. the cerebral peduncle,posterior limb of the internal capsule and corona radiata, than PVL infants without CP. There were deceased FA values, specifically along the CST, in the bilateral SCP group, but not in the No-CP group. Meanwhile, the posterior thalamic radiation, and the splenium of corpus callosum were damaged both in SCP and No-CP PVL infants due to immature blood vessels, pressure passive circulation, and selective vulnerability of preoligodendrocytes4. These results provide additional evidence that lesions in the CST may be the crucially damaged WM in bilateral SCP related to motor impairment. All these suggest that the CST may play a more important role than the other WM tracts in causing motor impairment.Conclusion
Lower FA in the CST maybe a prerequisite and biomarker for identifying and predicting the outcome of SCP in infants with PVL.Acknowledgements
This work was supported by the National Key Research and Development Program of China (2016YFC0100300), National Natural Science Foundation of China (No.81171317, 81471631), and the 2011 New Century Excellent Talent Support Plan of the Ministry of Education, China (NCET-11-0438).References
1 Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009; 8(1):110-124. 2 Beaino G, Khoshnood B, Kaminski M, et al. Predictors of cerebral palsy in very preterm infants: the EPIPAGE prospective population-based cohort study. Dev Med Child Neurol. 2010;52(6):e119-125. 3 Hadders-Algra M. Early diagnosis and early intervention in cerebral palsy. Front Neurol. 2014;5:185. 4 Hoon A H, Vasconcellos Faria A. Pathogenesis, neuroimaging and management in children with cerebral palsy born preterm. Dev Disabil Res Rev. 2010;16(4):302-312. ent.Figures
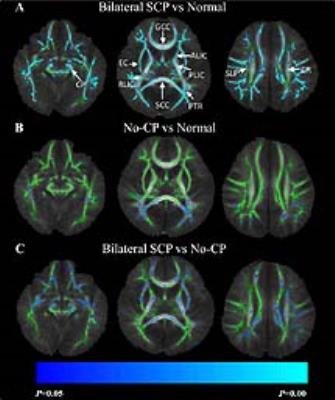
Figure 1: Group comparisons of fractional
anisotropy (FA) values between
SCP, No-CP, and control groups using tract-based spatial statistics (TBSS).
Color bars represent the range of p values from 0-0.05. Blue and light blue
regions show significantly decreased FA (P<0.05) in SCP (A) or No-CP group (B) relative to controls, and the SCP group relative to No-CP
group (C). Abbreviations: cerebral peduncle (CP); anterior and posterior limb of internal capsule
(ALIC& PLIC);
retrolenticular part of internal capsule (RPIC); posterior thalamic radiation
(PTR); corona radiata (CR); superior longitudinal fasciculus (SLF); external
capsule (EC); genu and splenium of corpus callosum (GCC& SCC).
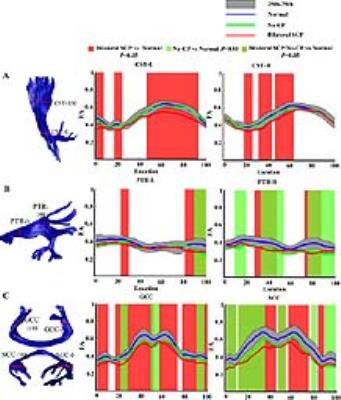
Figure 2: Tract profiles in corticospinal tract (CST) (A), posterior thalamic radiation (PTR)
(B), genu and splenium of corpus
callosum (GCC& SCC)(C) between SCP, No-CP, and control groups. For each tract, the fractional anisotropy (FA) values
along the core fiber (y-axis) are plotted for 100 equidistant locations
(x-axis) between two defining ROIs. The blue, red and green line respectively
represent the mean FA value in the control,
SCP and No-CP groups. The dark gray band shows 25th and 75th percentiles of the
control group. The red , green ,and yellow-green shades respectively represent
the significant difference in FA between groups (P<0.05).